Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Ionizing radiation therapy is an important component of cancer treatment. Researchers provide a summary of the latest advancements, clinical use, and limitations of radiation therapy. Moreover, researchers explored how radiation affects the body’s natural defense system, which plays a crucial role in fighting cancer. The immune responses triggered by radiation therapy help the body eliminate tumors naturally.
- radiation therapy
- DNA damage
- dendric cells
- NK cells
- macrophages
1. Introduction
Cancer remains the leading cause of death globally. According to the American Cancer Society (ACS), in 2023, an estimated 1,958,310 new cancer cases and 609,820 deaths from all cancers are anticipated to occur in the United States [1]. Cancer incidences increased for prostate cancer (PCa) by 3% annually from the year 2014 through 2019, following two decades of decline, translating to an additional 99,000 new cancer cases [1]. RT is one of the most effective forms of oncology care and continues to play a major role in the treatment of various types of malignancies. The emergence of advanced technologies has propelled a revolution in the field of RT, empowering clinicians with tools and techniques to deliver accurate and safe delivery of radiation doses to tumor cells. The advancement in intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT) and proton therapy have revolutionized the field. These technological advancements have resulted in increasingly conformal radiation treatments.
Ionizing radiation (IR) promotes primary effects on DNA structure by directly inducing DNA strand breaks, particularly double-strand breaks (DSBs), and indirectly induces secondary effects by ionizing water molecules to produce reactive oxygen species (ROS) [2][3]. ROS oxidize lipids and proteins and also induce various forms of DNA damage, including the generation of oxidized bases and single-strand breaks (SSBs) in the radiated cells/tissue, ultimately leading to cellular apoptosis [4]. Furthermore, IR also promotes clustered DNA damage and induces covalent inter- and intra-strand cross-linking [5]. Additionally, there is a growing recognition of the immune response generated by RT. Researchers focus on the implications of the innate myeloid and lymphoid lineages in anti-tumorigenic processes induced by RT and the use of various types of RT methods in cancer treatment [6][7]. Additionally, researchers also explore key strategies for enhancing the efficacy of RT while maintaining innate immunity during cancer therapy.
RT is an essential component of personalized medicine. It promotes immune suppression by inducing toxicity in bone marrow cells [8] and peripheral blood lymphocytes [9]. Additionally, RT activates innate immune systems and leads to bystander effects [10]. In the peripheral blood, dendritic cells, macrophages, and NK cells play a crucial role in regulating innate immunity and determining the efficacy of radiation therapy.
2. Innate Immunity
The immune system is classified into two categories: innate and adaptive immunity. Innate immunity is largely composed of myeloid/macrophages, natural killer (NK) cells, and dendritic cells. The innate immune system constitutes the first line of defense against invading microbial pathogens and recognizes the pathogens through pattern recognition receptors (PPRs) [11][12][13][14][15]. PPRs can detect conserved structures on pathogens termed pathogen-associated molecular patterns (PAMPs) [12]. However, recent findings suggest that the induction of immune effectors also commonly occurs in the absence of pathogen infection, which is termed sterile inflammation. Sterile inflammation is commonly found in RT-induced innate immune responses. PPRs also detect Damage Associated Molecular Patterns (DAMPs) [16][17] that originate within the damaged cell itself. The innate immune system initiates an immune response following the detection of DAMPs, which signals the status of tissue or cell damage or danger events. Innate immunity is activated by antigens and different immune cells, including dendritic, mast, natural killer (NK) cells, macrophages, monocytes, and granulocytes, to maintain the immune system. Adaptive immunity is mediated by lymphocytes such as T and B cells and is characterized by immunological memory cells that allow a long-lasting response. The effect of RT on adaptive immunity has been extensively discussed in the literature.
Role of RT in Priming the Innate Immune Response
RT induces apoptosis, which triggers DAMPs. Examples of DAMPs include the extracellular release of high mobility group box1, production of cytokines such as type I interferon (IFN-1), release of nuclear (nDNA) and mitochondrial DNA (mtDNA) to cytoplasm, and production of reactive oxygen species (ROS) or free radicles. These signals induce a series of chemical and immunological reactions that affect immunity. Mitochondria contain numerous potent immunostimulatory DAMPs, including mitochondrial DNA (mtDNA), ATP [18] and ROS. Mitochondrial DAMPs engage the innate immune macrophages or neutrophils [19] upon release to the cytosol or into the extracellular environment. The release of mtDNA into the cytosol activates PPRs to trigger a variety of innate immune responses. One such PPR is the DNA sensor cyclic GMP-AMP (cGAM) synthase, which binds cytosolic double-strand DNA (dsDNA) derived from mitochondria. This results in the generation of the second messenger cGAMP and activates the cGAS-STING pathway at the endoplasmic reticulum, leading to the recruitment of the tank binding kinase 1 (TBK1) and activation of the IFN signaling pathway [20]. Another DAMP is mitochondrial ATP, the key transporter of chemical energy. Recently, in several models, it has been shown that IR causes the release of ATP from tumor cells and activates DC cells [21]. ATP binds to P2X7 on DC cells, leading to the activation of NLRF3 inflammasomes [22].
3. Mechanisms of Radiation-Induced Innate Immune Cell Activation
3.1. Dendritic Cells
Myeloid cells constitute a highly diverse population that is comprised mainly of dendric cells (DCs), monocytes and macrophages [23]. Dendritic cells play a crucial role in host immunity by promoting innate inflammatory responses to environmental or damage stimuli [24]. TNF-α and IL-1β are proinflammatory signaling molecules that are upregulated in response to IR. These molecules, in turn, activate antigen-presenting innate immune cells, including dendritic cells [25][26][27][28][29]. Dendritic cells are specialized antigen-presenting cells that play a crucial role in T-cell activation following RT-induced damage in tumor cells. Dendritic cells recognize DAMPs via specific receptors and matured dendritic cells [30] stimulate cytotoxic CD8+ T cells by antigen presentation and release of activating cytokines, thereby enhancing RT treatments. The intensity of radiation doses and the number of doses determines the immunogenic action of dendritic cells in RT. For example, repeated low radiation doses in a murine mammary carcinoma model create cytosolic DNA in tumor cells, activating the cGAS-STING pathway and the release of IFN-γ and subsequent T-cell activation [13][31][32] (Figure 1A,B). RT sensitivity depends in part on DNA exonuclease called 3′ repair exonuclease 1 (Trex1). Trex1 cleaves the RT-induced cytosolic DNA, thereby abrogating IFN-β production through the cGAS-STING pathway. The Trex1 level does not increase in response to multiple smaller fractions of radiation (8 Gy, three times); rather, it induces greater IFN-β production and activation of Bat3-dependent dendritic cells, leading to enhanced T cell responses. Compared to a single fraction of high-dose radiation, the induction of Trex1 in multi-low-dose RT is efficient and suggests that a fractionated low-dose of RT likely plays a role in enhancing immunogenicity [31][33].
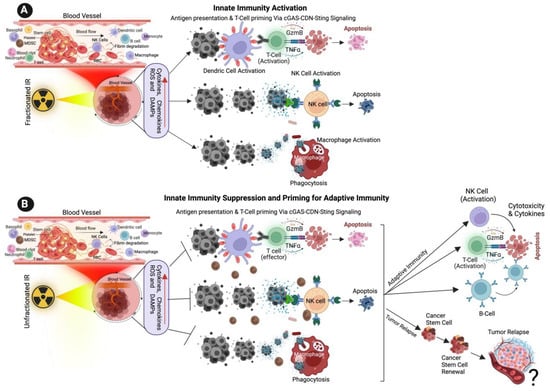
Figure 1. Effect of Ionizing Radiation on Innate Immune Activation. (A) The illustration shows the low dose of fractionated RT induces the production of ROS, cytokines, chemokines, and damage-associated molecular patterns (DAMPs) from cancer cells, DAMPs promoting the activation of innate immune dendritic cells, nature killing cells (NK cells) and macrophages. Activated dendric cells present tumor antigens and are primed for T-cell activation. Activated NK cells promote cytotoxic effects on tumor cells, and activated macrophages destroy cancer cells via active phagocytosis. (B) The cartoon illustration shows that a high dose of unfractionated radiation induces the production of ROS and DAMPs from cancer cells, inactivating innate immune dendritic cells, macrophages, and NK cells, promoting immune escape and tumor relapse. Unfractionated radiation also promotes adaptive immune cell activation via T-cells, NK cells and B-cells and promotes intra-tumoral immunity. ? indicates may or may not happen, arrow indicates the next step.
3.2. Natural Killer Cells
NK cells effector lymphocytes play a crucial role in regulating innate immune responses, combating microbial infections, and controlling cancer. While IR has been shown to have a significant impact on NK cells, the underlying mechanisms behind this effect remain unclear [34]. NK cells are innate immune lymphocytes that can destroy target tumor cells by producing toxic and immunoregulatory cytokines [35][36]. IR has a significant effect on modifying NK cells. Previous studies have demonstrated that IR enhances the immune response by augmenting the antigenicity and adjuvanticity of malignant cells and by interacting with the tumor microenvironment (TME) [37]. Low-dose ionizing radiation activates NK cell functions, while high-dose ionizing radiation particularly impairs NK cell function (Figure 1A,B); however, this impairment can be reversed by interleukin-2 (IL-2) pretreatment [38][39][40]. Low-dose ionizing radiation at 75–150 mGy increases the secretion of NK cell effector proteins, such as IFN-γ and TNF-α [34][41]. Similarly, low-dose total-body irradiation (0.1 or 0.2 Gy X-ray) results in the suppression of experimental tumor metastases along with the stimulation of NK cell cytolytic functions in tumor-bearing rates [42][43].
Studies have identified that low-dose ionizing RT (LDIRT) can increase the immune response in vivo [41] with IFN-γ and TNF-α in the cultured medium of NK cells in response to LDIRT, and in addition, the P38 inhibitor (SB203580) drastically suppressing the NK cell cytotoxicity, cytokine levels, FasL and perforin [41][44]. Ames et al. (2015) identified that ex vivo NK cells are activated following low dose IL-2 and IL-15 and presented an increased ability to mitigate solid tumor cells in vitro and in vivo following RT [45]. A similar study reported that the presence of the cytotoxic effect in NK cells was boosted following RT in canine models of sarcoma, and the results from a clinical are progressing with possible abscopal effects. In general, NK cells produce perforin (Prf1) and granzyme B (GzmB) and induces cancer cell apoptosis (Figure 1) [46][47]. It is also observed that dendritic cells (DC) activate NK cells and promote tumor cell apoptosis [48]. A recent study utilized a reverse translational approach and revealed that NK cells play a role in immune enhancement through a CXCL8/IL-8-dependent mechanism in response to radiation [49]. Furthermore, the study suggests that NF-κB and mTOR mediate the secretion of chemokines that facilitates the infiltration of NK cells into tumor cells. Additionally, the study highlights that higher doses of radiation promote the transfer of adoptive NK cells and improve tumor control [49].
3.3. Macrophages
Macrophages play a crucial role in various aspects of immunity, including infiltrating the TME. Macrophages belong to the myeloid family and originate from circulating bone marrow-derived monocyte precursors. Macrophages are highly plastic cells that undergo significant changes in their function depending on the environmental cues in the TME, exerting a dual function on tumorigenesis by either antagonizing the cytotoxic activity of immune cells or enhancing the antitumor responses (Figure 1A,B). Macrophages are classified into two different phenotypes, M1 and M2. M1 macrophages are called classically activated macrophages in response to pathogens and take part in the immune response. M2 macrophages are known as alternatively activated macrophages involved in wound repair and have an anti-inflammatory role. Following recruitment, the monocyte precursor cells differentiate into macrophages in the tissue. The matured macrophages polarize to functionally different phenotypes in response to microenvironmental challenges in TME in tumor cells. Tumor-associated macrophages (TAMs), a major stromal component of TME, resemble the M2-polarized macrophages [50][51][52]. M1 macrophages are also involved in antitumor immunity, while M2 macrophages exert pro-tumorigenic activities.
Macrophages are recruited to the damaged or injured sites following the radiation exposure, where they carry out their phagocytic function [53][54][55]. Macrophage responses to RT range from promoting tumor growth to enhancing the immunogenic response, depending on the tumor type, environment, IR and dose, and fractionation. Inducible nitric oxide synthase (iNOS)+ M1-like macrophages undergo differentiation in response to local low-dose ionizing radiation, allowing the recruitment of tumor-specific T-cells and tumor regression in human pancreatic carcinomas [51][56]. Irradiated cells induce cytokine secretion and hypoxia within the damaged tissue, and the activation of the transcription factor HIF1-α has been shown to contribute to the recruitment of macrophages towards the immunosuppressive phenotype. Activated macrophages can directly destroy cancer cells by enhancing the phagocytosis of tumor cells through antibody-dependent cellular cytotoxicity. Alternatively, by secreting toxic/harmful molecules such as cytokines or tumor necrosis factors TNF or nitric oxide and promote cytolysis of cancer cells. The indirect killing of tumor cells involves the recruitment of immune cells, such as cytotoxic T-cells (Figure 1) [57][58][59].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15153972
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Buatti, J.M.; Rivero, L.R.; Jorgensen, T.J. Radiation-induced DNA single-strand breaks in freshly isolated human leukocytes. Radiat. Res. 1992, 132, 200–206.
- Mahaney, B.L.; Meek, K.; Lees-Miller, S.P. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem. J. 2009, 417, 639–650.
- Borrego-Soto, G.; Ortiz-Lopez, R.; Rojas-Martinez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432.
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60.
- Dennstadt, F.; Treffers, T.; Iseli, T.; Panje, C.; Putora, P.M. Creation of clinical algorithms for decision-making in oncology: An example with dose prescription in radiation oncology. BMC Med. Inform. Decis. Mak. 2021, 21, 212.
- Leech, M.; Katz, M.S.; Kazmierska, J.; McCrossin, J.; Turner, S. Empowering patients in decision-making in radiation oncology—Can we do better? Mol. Oncol. 2020, 14, 1442–1460.
- Akeem, S.; Lukman, O.; Eltahir, K.; Fatai, O.; Abiola, B.; Khadijat, O. Bone Marrow and Peripheral Blood Cells Toxicity of a Single 2.0 Gy Cobalt(60) Ionizing Radiation: An Animal Model. Ethiop. J. Health Sci. 2019, 29, 195–202.
- Scott, D.; Lyons, C.Y. Homogeneous sensitivity of human peripheral blood lymphocytes to radiation-induced chromosome damage. Nature 1979, 278, 756–758.
- Marin, A.; Martin, M.; Linan, O.; Alvarenga, F.; Lopez, M.; Fernandez, L.; Buchser, D.; Cerezo, L. Bystander effects and radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 12–21.
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801.
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379.
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149.
- Jang, J.H.; Shin, H.W.; Lee, J.M.; Lee, H.W.; Kim, E.C.; Park, S.H. An Overview of Pathogen Recognition Receptors for Innate Immunity in Dental Pulp. Mediat. Inflamm. 2015, 2015, 794143.
- Shertzer, H.G.; Sainsbury, M. Intrinsic acute toxicity and hepatic enzyme inducing properties of the chemoprotectants indole-3-carbinol and 5,10-dihydroindeno indole in mice. Food Chem. Toxicol. 1991, 29, 237–242.
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286.
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049.
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173.
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal 2015, 23, 1329–1350.
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557.
- O’Carroll, P.W.; Cahn, M.A.; Auston, I.; Selden, C.R. Information needs in public health and health policy: Results of recent studies. J. Urban Health 1998, 75, 785–793.
- Aymeric, L.; Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Martins, I.; Kroemer, G.; Smyth, M.J.; Zitvogel, L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010, 70, 855–858.
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopes, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021, 184, 5338–5356.e21.
- Price, J.D.; Tarbell, K.V. The Role of Dendritic Cell Subsets and Innate Immunity in the Pathogenesis of Type 1 Diabetes and Other Autoimmune Diseases. Front. Immunol. 2015, 6, 288.
- Hallahan, D.E.; Spriggs, D.R.; Beckett, M.A.; Kufe, D.W.; Weichselbaum, R.R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. USA 1989, 86, 10104–10107.
- Hong, J.H.; Chiang, C.S.; Tsao, C.Y.; Lin, P.Y.; McBride, W.H.; Wu, C.J. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int. J. Radiat. Biol. 1999, 75, 1421–1427.
- Ishihara, H.; Tanaka, I.; Nemoto, K.; Tsuneoka, K.; Cheeramakara, C.; Yoshida, K.; Ohtsu, H. Immediate-early, transient induction of the interleukin-1 beta gene in mouse spleen macrophages by ionizing radiation. J. Radiat. Res. 1995, 36, 112–124.
- Nemoto, K.; Ishihara, H.; Tanaka, I.; Suzuki, G.; Tsuneoka, K.; Yoshida, K.; Ohtsu, H. Expression of IL-1 beta mRNA in mice after whole body X-irradiation. J. Radiat. Res. 1995, 36, 125–133.
- McBride, W.H.; Chiang, C.S.; Olson, J.L.; Wang, C.C.; Hong, J.H.; Pajonk, F.; Dougherty, G.J.; Iwamoto, K.S.; Pervan, M.; Liao, Y.P. A sense of danger from radiation. Radiat. Res. 2004, 162, 1–19.
- Porkolab, V.; Chabrol, E.; Varga, N.; Ordanini, S.; Sutkeviciu Te, I.; Thepaut, M.; Garcia-Jimenez, M.J.; Girard, E.; Nieto, P.M.; Bernardi, A.; et al. Rational-Differential Design of Highly Specific Glycomimetic Ligands: Targeting DC-SIGN and Excluding Langerin Recognition. ACS Chem. Biol. 2018, 13, 600–608.
- Dar, T.B.; Henson, R.M.; Shiao, S.L. Targeting Innate Immunity to Enhance the Efficacy of Radiation Therapy. Front. Immunol. 2018, 9, 3077.
- Vatner, R.E.; Janssen, E.M. STING, DCs and the link between innate and adaptive tumor immunity. Mol. Immunol. 2019, 110, 13–23.
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618.
- Chen, J.; Liu, X.; Zeng, Z.; Li, J.; Luo, Y.; Sun, W.; Gong, Y.; Zhang, J.; Wu, Q.; Xie, C. Immunomodulation of NK Cells by Ionizing Radiation. Front. Oncol. 2020, 10, 874.
- Crinier, A.; Narni-Mancinelli, E.; Ugolini, S.; Vivier, E. SnapShot: Natural Killer Cells. Cell 2020, 180, 1280–1280.e1.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510.
- Wennerberg, E.; Vanpouille-Box, C.; Bornstein, S.; Yamazaki, T.; Demaria, S.; Galluzzi, L. Immune recognition of irradiated cancer cells. Immunol. Rev. 2017, 280, 220–230.
- Ina, Y.; Sakai, K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: Analysis of immune cell populations and surface molecules. Int. J. Radiat. Biol. 2005, 81, 721–729.
- Lacoste-Collin, L.; Jozan, S.; Cances-Lauwers, V.; Pipy, B.; Gasset, G.; Caratero, C.; Courtade-Saidi, M. Effect of continuous irradiation with a very low dose of gamma rays on life span and the immune system in SJL mice prone to B-cell lymphoma. Radiat. Res. 2007, 168, 725–732.
- Zarcone, D.; Tilden, A.B.; Lane, V.G.; Grossi, C.E. Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood 1989, 73, 1615–1621.
- Yang, G.; Kong, Q.; Wang, G.; Jin, H.; Zhou, L.; Yu, D.; Niu, C.; Han, W.; Li, W.; Cui, J. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother. Radiopharm. 2014, 29, 428–434.
- Cheda, A.; Wrembel-Wargocka, J.; Lisiak, E.; Nowosielska, E.M.; Marciniak, M.; Janiak, M.K. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat. Res. 2004, 161, 335–340.
- Hashimoto, S.; Shirato, H.; Hosokawa, M.; Nishioka, T.; Kuramitsu, Y.; Matushita, K.; Kobayashi, M.; Miyasaka, K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat. Res. 1999, 151, 717–724.
- Chini, C.C.; Boos, M.D.; Dick, C.J.; Schoon, R.A.; Leibson, P.J. Regulation of p38 mitogen-activated protein kinase during NK cell activation. Eur. J. Immunol. 2000, 30, 2791–2798.
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Smith, R.C.; Monjazeb, A.M.; Chen, M.; Murphy, W.J. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology 2015, 4, e1036212.
- Terme, M.; Ullrich, E.; Delahaye, N.F.; Chaput, N.; Zitvogel, L. Natural killer cell-directed therapies: Moving from unexpected results to successful strategies. Nat. Immunol. 2008, 9, 486–494.
- Yoon, S.R.; Kim, T.D.; Choi, I. Understanding of molecular mechanisms in natural killer cell therapy. Exp. Mol. Med. 2015, 47, e141.
- Borg, C.; Taieb, J.; Terme, M.; Maruyama, K.; Flament, C.; Angevin, E.; Zitvogel, L. . Bull. Cancer 2003, 90, 699–705.
- Walle, T.; Kraske, J.A.; Liao, B.; Lenoir, B.; Timke, C.; von Bohlen Und Halbach, E.; Tran, F.; Griebel, P.; Albrecht, D.; Ahmed, A.; et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci. Adv. 2022, 8, eabh4050.
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904.
- Gomez, V.; Mustapha, R.; Ng, K.; Ng, T. Radiation therapy and the innate immune response: Clinical implications for immunotherapy approaches. Br. J. Clin. Pharmacol. 2020, 86, 1726–1735.
- Shi, X.; Shiao, S.L. The role of macrophage phenotype in regulating the response to radiation therapy. Transl. Res. 2018, 191, 64–80.
- Meziani, L.; Mondini, M.; Petit, B.; Boissonnas, A.; Thomas de Montpreville, V.; Mercier, O.; Vozenin, M.C.; Deutsch, E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 2018, 51, 1702120.
- Travis, E.L. The sequence of histological changes in mouse lungs after single doses of x-rays. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 345–347.
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040.
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602.
- Aminin, D.; Wang, Y.M. Macrophages as a “weapon” in anticancer cellular immunotherapy. Kaohsiung J. Med. Sci. 2021, 37, 749–758.
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516.
- Salah, A.; Li, Y.; Wang, H.; Qi, N.; Wu, Y. Macrophages as a Double-Edged Weapon: The Use of Macrophages in Cancer Immunotherapy and Understanding the Cross-Talk between Macrophages and Cancer. DNA Cell Biol. 2021, 40, 429–440.
This entry is offline, you can click here to edit this entry!
