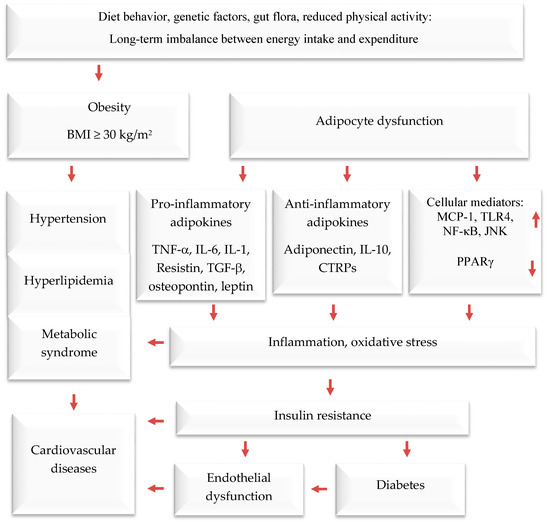
Obesity is a serious public health threat, contributing to many chronic diseases worldwide, particularly type 2 diabetes (T2D), cardiovascular disease (CVD), hypertension, and certain cancers [
1,
2,
3]. In recent decades, cultural globalization and urbanization have led to a shift towards a lifestyle with reduced physical activity and that involves consuming more foods high in refined carbohydrates, salt, saturated fats, and proteins, while eating fewer fruits, vegetables, and wild edible plants (WEPs). Obesity causes adipocyte hypertrophy and hyperplasia, leading to molecular and cellular changes that can affect systemic metabolism. This can result in metabolic syndrome and comorbidities such as T2D, CVD, hypertension, and endothelial dysfunction [
4,
5,
6]. Additionally, the increased weight associated with obesity can cause mechanical problems such as osteoarthritis and sleep apnea, affecting quality of life. Numerous studies have demonstrated that obesity elevates the risk of contracting communicable diseases, especially viral infections [
7,
8] (
Figure 1).
Adipose tissue is responsible for more than just storing fat and balancing energy. It also produces various molecules that can affect the immune system, such as adiponectin, adipokines, cytokines, and chemokines. These molecules help regulate metabolism and inflammation both locally and throughout the body [
21,
22,
23]. Brown and white adipose and tissues are the main types of adipose tissue. The former burns energy to maintain body temperature. On the other hand, white adipose tissue functions as an energy store, offers insulation against the cold, and provides cushioning to protect the body. Additionally, it serves as an endocrine organ [
24,
25,
26]. It usually produces anti-inflammatory mediators; when cells become too large, they can release pro-inflammatory hormones and cytokines. These include, among others, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), angiotensinogen, transforming growth factor-beta (TGF-β), adiponectin, resistin, monocyte chemoattractant protein-1 (MCP-1), and leptin (a hormone that increases inflammation and reduces the release of adiponectin, which reduces inflammation). Visceral fat tissue has a higher rate of fat breakdown and macrophage infiltration and releases more IL-6 and MCP-1 than subcutaneous fat tissue. As obesity levels rise, monocytes migrate into adipose tissue and differentiate into macrophages [
27,
28], which release pro-inflammatory agents that contribute to chronic low-grade inflammation. The latter reduces insulin sensitivity, leading to high blood glucose levels and eventually T2D and related diseases. According to recent studies, fat tissue around the waist releases pro-inflammatory mediators [
29,
30], which impair insulin sensitivity in obese persons.
2. Prevention and Treatment of Obesity by Mediterranean Dietary Compounds and Wild Plants
In addition to using the whole herb or its extracts, numerous scientific in vitro and clinical trials have confirmed the anti-obesity activities of phytochemicals [
33,
35,
36,
37]. The pharmacological mechanisms of the anti-obesity effects of active compounds from pomegranate, citrus fruits, rosemary, black seeds, cumin, ginger, olive leave/oil, turmeric, cinnamon, fenugreek, and garlic have been evaluated through tests on cells, animals, and humans [
33,
34,
35,
36,
37]. Dietary compounds, medicinal plants, and phytochemicals generated from them were reported to fight obesity by affecting different processes in the body. These include the blocking of pancreatic lipase and glucosidase, the suppression of hunger, the stimulation of thermogenesis and lipid metabolism, and the inhibition of fat breakdown and adipogenesis (
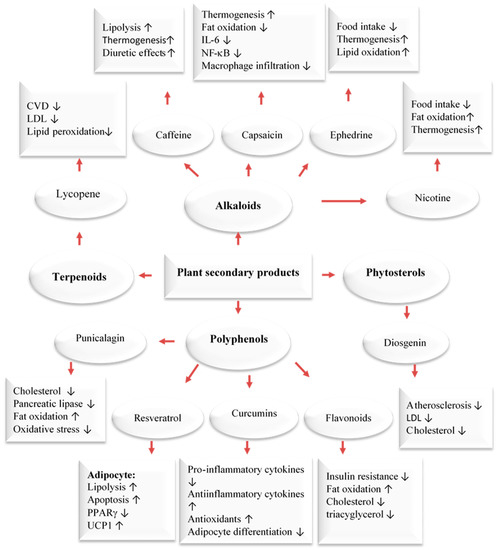
Figure 2). Phytochemicals have been found to target fat cells in several ways, including inhibiting fat cell formation, promoting fat breakdown, and reducing energy intake while increasing energy expenditure (
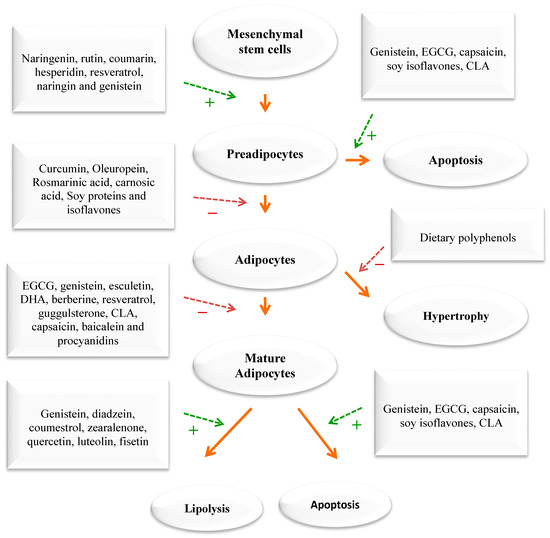
Figure 3). These findings come mainly from laboratory and animal studies. However, more high-quality research is needed to confirm the effectiveness of these phytochemicals in humans. Some examples of polyphenols include quercetin, myricetin, diadzein, genistein, cyanidin, luteolin, apigenin, kaempferol, proanthocyanidin, xanthohumol, and epigallocatechin gallate (EGCG). Numerous scientific studies demonstrated that these compounds exhibit weight-reducing and anti-obesity effects [
33,
35,
36,
37,
38,
39] (
Figure 2).
Figure 2. Major classes of plant secondary metabolites and their anti-obesity mechanisms [
39]. Upwards: Activation; downwards arrows: Inhibition.
Figure 3. The impact of phytochemicals on adipogenesis. Green dashed arrows: Activation; red dashed arrows: Inhibition.
3. Wild Edible Plants and Their Active Compounds
Due to their rich content in plant secondary metabolites, including polyphenols and terpenoids, WEPs are prime candidates for use in nutraceuticals or functional foods. The Mediterranean area is renowned for its abundant variety of WEPs that are edible and form a vital component of this diet. Local communities have long acknowledged the nutritional, protective, and medicinal benefits of these plants, even before their advantages were scientifically validated. In the eastern Mediterranean, WEPs continue to be valued as healthy food sources and are often collected by women as a means of subsistence and income generation in rural areas with limited economic opportunities [
40,
41,
42]. The scientific community has recently shown an increased interest in traditional Arab-Islamic herbal medicine, particularly in its potential for treating metabolic and chronic diseases [
38,
39]. This practice, known as Greco-Arab medicine, remains dominant in the Mediterranean region as well as in many Arab and Islamic countries. According to surveys, the Middle East is home to over 2600 species of plants, of which more than 700 are utilized in treating various ailments. Currently, Arab traditional medicine uses fewer than 200–250 plant species to treat various diseases. Many of these species are WEPs that have been assessed in cell culture, in vivo, and in clinical trials and were found to contain pharmacology active compounds [
43]. WEPs have similar effects as medicinal plants. They can modulate appetite, lipase activity, thermogenesis and fat synthesis and degradation, satiation, adipogenesis, and adipocyte apoptosis. Additionally, targeting adipocyte growth and differentiation with diverse medicinal plants/diet is a significant strategy for devising new anti-obesity drugs that can intervene in preadipocytes, maturing preadipocytes, and mature adipocytes.
Many plant extracts from the Mediterranean region and their natural compounds have been tested for their anti-obesity effects in the past ten years [
33,
34,
41]. Specifically, 31 plant species showed the ability to block the pancreatic lipase enzyme. The flavonoid rutin (found in citrus and in a wide range of herbs) and the phenolic acids p-coumaric (found in a wide variety of herbs) and ferulic acids (an antioxidant found in a wide range of plants), which are three bioactive compounds, were found to be successful in blocking this enzyme. It was also shown that trans-anethole and resveratrol, two natural compounds, work by stimulating the brown fat tissue [
41].
In addition, wild cranberries can influence fat cell formation, and blueberries have shown a remarkable ability to lower blood lipids. In addition, 28 plants from the Mediterranean region and 21 active substances from them have shown promising effects on sugar metabolism. Many studies have examined how plant extracts and pure substances can block digestive enzymes related to obesity and T2D (α-amylase, α-glucosidase, and pancreatic lipase). For example, the effects of 18 WEPs that people eat in southern Italy’s Calabria region on pancreatic lipase, an enzyme that breaks down fats, were tested in a recent study [
44,
45]. Nine of the plant extracts could inhibit the enzyme at a concentration of less than 10 mg/mL. The leaves of purslane
(Portulaca oleracea) and bladder campion
(Silene vulgaris), extracted with water and ethanol, had the strongest inhibition, with 5.48 mg/mL and 6.02 mg/mL needed to block half of the enzyme activity, respectively. Among the plants from the mint family (Lamiaceae), spearmint
(Mentha spicata) and rosemary (
Rosmarinus officinalis) showed inhibition, with 7.85 mg/mL and 7.00 mg/mL required, respectively. The most effective plants from the sunflower family (Asteraceae) and the cabbage family (Brassicaceae) were common sowthistle
(Sonchus oleraceus) (9.75 mg/mL) and perennial wall-rocket
(Diplotaxis tenuifolia) (7.76 mg/mL), respectively.
Many phytochemicals can help reduce weight by decreasing the absorption of lipids and inhibiting pancreatic lipase. For example, green tea contains catechins and saponins, such as EGCG, which have this effect. Pomegranate (
Punica granatum) contains punicalagin, ellagic acid, and anthocyanins; rosemary has rosmarinic acid and carnosic acid; black seed contain thymoquinone; and soybean contains proteins and isoflavones that can also help with weight reduction [
33,
35]. However, most of the studies on how plant extracts affect the body were conducted in vitro or on animals. In addition, many new substances and natural products from plants were found to block pancreatic lipase, an enzyme that breaks down fat, better than orlistat, a common drug for weight loss. Some of these extracts have strong effects on fat digestion because of their compounds like polyphenols, saponins, and terpenes. Many natural products that stop pancreatic lipase are being tested on animals or cells, but none of them have been tested on humans yet. Sometimes, it is hard to apply the findings from these tests to clinical use, because they may not work as well in practice. Therefore, the main problem with these studies is that even though many plant chemicals are stronger than orlistat, we do not know how safe they are compared to orlistat.
When energy intake consistently exceeds energy expenditure, the excess energy is mostly stored as triglycerides in fat tissue. An increase in adipose tissue mass can result from an increase in cell size, cell number, or both. The cellular pathways that control the growth of pre-adipose cells, adipose differentiation, and lipogenesis in adipocytes were thoroughly investigated [
1,
3,
4] (
Figure 4). Recently, the differentiation of pre-adipocytes has garnered research attention and has been investigated through in vitro adipogenesis models, including the 3T3-L1 cell line [
46,
47,
48,
49]. Many published reports focused on the effects of medicinal plants and their active compounds on adipocyte life cycle. Curcumin (from turmeric), oleuropein (from olive oil), thymoquinone (from black seeds), rosmarinic acid (from rosemary), resveratrol (from grapes), punicalagin (from pomegranate), coumestrol (from soybeans), quercetin (found in many fruits, flowers, and vegetables), Luteolin (found in many fruits, vegetables, and medicinal herbs), and fisetin (found in many fruits and vegetables such as strawberry, apple, and onion) were reported to affect the adipogenesis (
Figure 3).
Figure 4. Anti-obesity and its related disease mechanisms of plants and their active compounds. Dashed arrows: Indirect effect; solid arrows: Direct effect; Upwards: Activation; downwards arrows: Inhibition.
Our bodies maintain an equilibrium of body mass, specifically body lipids, through the coordinated functioning of the autonomic, nervous, endocrine, and metabolic systems. This equilibrium is based on an ‘ideal’ level determined by the central nervous system weight loss. This system can cause 80% of the weight to be regained. Brown adipose tissue plays a key role in energy expenditure by generating heat through thermogenesis. This process is regulated by a metabolic pathway involving the hormone leptin and the protein PGC-1𝛼. This pathway is responsible for burning fat and generating heat, but it can also make it difficult to sustain weight loss due to opposition from the body’s equilibrium. Thermogenesis is a process that mainly takes place in brown adipose tissue but also in beige cells located in white adipose tissue. This process, which is beneficial for preventing obesity, has been shown to be accelerated by capsaicin, caffeine, ephedrine, resveratrol, EGCG, gingerol, and oleuropein. It has been proposed that these compounds could be used to treat obesity and being overweight. For example, caffeine produces thermogenic effects by preventing the degradation of cAMP caused by phosphodiesterase. Caffeine has been shown to decrease food intake and lipid storage. Human studies have also demonstrated that capsaicin, (an active component of chili peppers) and EGCG can increase thermogenesis. Capsaicin treatment stimulates the secretion of catecholamines from the adrenal medulla in a dose-dependent manner, resulting in a thermogenic effect. EGCG increases thermogenesis by inhibiting catechol methyl-transferase, an enzyme responsible for the degradation of norepinephrine [
33,
41].
4. The Mediterranean Diet and Its Active Compounds
The main ingredients of the Mediterranean diet (MedDiet) are among the most researched dietary components for treating and preventing various metabolic disorders and CVD. They are recognized for decreasing the risk of CVD, hypertension, T2D, being overweight/obesity, breast and colon cancers, asthma, and mental decline [
33,
34,
50]. The MedDiet, which is rich in antioxidants and has anti-inflammatory, hypotensive, and hypolipidemic properties, is a great alternative to a diet high in red meat for reducing the risk of CVD. Its positive effects are similar to those of standard drugs such as beta-blockers, aspirin, and angiotensin-converting enzyme inhibitors [
50,
51,
52,
53,
54,
55,
56]. However, it is not yet clear whether these benefits come from individual components of the diet or their combined and synergistic effects [
50,
51,
52,
53].
There is a lot of evidence that shows a lower risk of mortality from CVD, T2D, specific types of cancer, and cognitive problems when following a MedDiet [
53,
54]. A comprehensive review of 27 meta-analyses based on 70 cohort studies found 34 different ways to measure the MedDiet [
57]. There are various ways to evaluate the MedDiet [
58,
59], and all of them involve the common food groups that define this dietary pattern. These include a high intake of fruits, vegetables, nuts, legumes, fish, whole grain cereals, and extra virgin olive oil; a moderate consumption of alcohol, preferably red wine; and a low consumption of dairy products, red meat, and processed meat.
Recent meta-analyses of observational studies have consistently shown that following a MedDiet is associated with positive health outcomes [
60,
61,
62]. Critical reviews of both observational studies and RCTs have also confirmed the health benefits of this diet [
63,
64,
65,
66]. A recent Cochrane review concluded that the evidence supporting the effectiveness of the MedDiet in preventing CVD is only of low to moderate certainty. A recent Cochrane review concluded that the evidence supporting the effectiveness of the MedDiet in preventing CVD is only of low to moderate certainty [
63]. Nutrition research RCTs are often limited by factors such as small sample sizes, high dropout rates, and short follow-up periods, whereas larger samples, lower dropout rates, and longer follow-up periods are needed to observe patient-relevant outcomes [
64]. Some discrepancies may arise from the use of varying definitions of the dietary pattern being studied. Inconsistencies may also result from differences in the diets being compared.
The health benefits of the MedDiet may be attributed to its impact on the composition and metabolism of the gut microbiota. A systematic review was recently conducted to investigate the effects of this diet on the gut microbiota, as observed in both observational studies and randomized controlled trials. While some research has indicated that a MedDiet may have a positive effect on certain microbiota, a systematic review found that this diet did not consistently alter the composition or metabolism of the microbiota. This inconsistency may be attributed to variations in methodology among studies, particularly in the composition of the MedDiet [
65].
5. The MedDiet and Cardiovascular Disease
A recent long-term RCT (CORDIOPREV study) from Córdoba, Spain, with a 7-year follow-up, compared the effects of the Mediterranean and low-fat diets on the secondary prevention of CVD based on cardiovascular outcomes in patients with coronary heart disease [
66]. The study involved 1002 patients, with 500 assigned to a low-fat diet group and 502 to a MedDiet group. The primary outcome was a composite of major cardiovascular events, including myocardial infarction, revascularization, ischaemic stroke, peripheral artery disease, and cardiovascular death. The primary endpoint occurred in 198 participants: 87 in the MedDiet group and 111 in the low-fat group. Multivariable-adjusted hazard ratios of the different models ranged from 0.719 to 0.753 in favor of the MedDiet. These effects were more evident in men, with primary endpoints occurring in 16.2% of the men in the MedDiet group versus 22.8% of the men in the low-fat diet group. These data suggest that, in terms of preventing major cardiovascular events in secondary prevention, the MedDiet was more effective than a low-fat diet [
66].
6. Anti-Obesity Effects of MedDiet Polyphenols and Their Possible Mechanisms of Action
The polyphenol rich components of the MedDiet are responsible for the health benefits of this diet. In general, polyphenols are classified into two groups: flavonoids and non-flavonoid polyphenols. Members of both groups can exist in their free form or combined with sugars or acylated sugars (glycosides) or amides, esters, and methyl ethers. Flavonoids include around 6000 different chemicals and are classified into several subgroups [
116,
117]. Hydrolysable tannins, lignans, stilbenes, and phenolic acids belong to non-flavonoid polyphenols that have a more complex structure [
118,
119,
120]. Regular consumption of polyphenols is associated with a lower blood pressure and adiposity, an improved lipid profile, as well as antioxidant and anti-inflammatory effects, all of which help protect against CVD [
81,
121,
122]. Polyphenols may contribute to weight loss through several mechanisms. These include promoting satiety, stimulating thermogenesis by activating brown fat, regulating fat tissue by inhibiting fat cell growth and encouraging fat cell apoptosis, and controlling the β-oxidation [
123,
124,
125] (
Figure 2).
Evidence regarding the impact of polyphenols on obesity and related complications in humans is inconsistent. This is due to variations in study designs, populations, intervention periods, and polyphenol supplements. A systematic review of five RCTs compared the MedDiet with low-fat diets, a low-carbohydrate diet, and the American Diabetes Association (ADA) diet [
126]. The results show that the MedDiet was more effective for weight loss than low-fat diets but had similar results to the other two interventions. It is unclear if following a traditional MedDiet leads to a reduction in body weight and waist circumference. A meta-analysis of 16 RCTs, however, found that greater adherence to the MedDiet resulted in more weight reduction when compared to a control diet [
126,
127]. The MedDiet may promote weight loss due to its high fiber content, low energy density, and low glycemic load. The impact on body weight was more significant when the MedDiet was combined with a calorie-restricted plan or increased physical activity. In T2D patients, the Mediterranean-style diet was found to decrease BMI compared to the control diets [
127,
128].
While some clinical trials have found that polyphenol-enriched foods can decrease body fat mass, they have not shown reductions in body weight, BMI, or waist circumference [
129]. In contrast, a recent study using a polyphenol supplement found significant reductions in these measures. Few studies have investigated the link between total dietary polyphenol intake and weight control. One long-term study found that a higher total polyphenol excretion was associated with a lower BMI, body weight, and waist circumference [
130,
131,
132]. In a 14-year longitudinal study of 4280 participants aged 55–69 in the Netherlands, it was found that women who had a higher intake of flavonoids experienced a lower increase in BMI [
133].
7. Anti-Obesity Effects of Carotenoids and Their Possible Mechanisms of Action
Many studies have found a link between obesity and low levels of carotenoids in the blood [
155,
156]. Carotenoids are a type of hydrophobic pigment present in vegetables and fruits, which cannot be synthesized by the human body. Consuming them has been linked to numerous health benefits for humans, including a reduction in overall mortality [
157]. One of their key characteristics is their ability to affect oxidative stress and inflammation by interacting with transcription factors [
158]. For instance, they can serve as precursors for bioactive derivatives that activate signaling through nuclear hormone receptors. These derivatives, such as retinoids or vitamin A derived from β-carotene, can activate retinoic acid receptors (RARs), which are a type of nuclear hormone receptor [
159]. Recent research has led to the discovery of various new metabolic pathways. These pathways are mediated through specific nuclear hormone receptor activation pathways, which were predicted and subsequently confirmed.
There is a strong negative correlation between body mass index (BMI) and the levels of all measured carotenoids in the blood. Additionally, many disorders associated with obesity, such as chronic low-grade inflammation and insulin resistance, also show a strong negative correlation with the levels of carotenoids in the blood [
160,
161,
162]. Consuming a diet high in fat can alter the functions of white adipose tissue, which can affect the way AMPK regulates the breakdown of fats and lipid metabolism in fat cells. By activating AMPK, it may be possible to reduce oxidative stress and inflammation. Consuming carotenoids, either through diet or supplements, has been shown to help reduce complications caused by a high-fat diet [
163]. Different types of carotenoids can stimulate the AMPK signaling pathway, activating enzymes, increasing the activity of transcription factors, promoting the conversion of white adipose tissue to brown, and inhibiting the formation of new fat cells. Carotenoids may also improve the levels of certain “homeostatic” factors, such as adiponectin, which may play a role in activating AMPK. Based on these findings, it is recommended that clinical trials be conducted to confirm the effects of carotenoids on the AMPK pathway in long-term treatments, particularly in cases of obesity [
163].
Several studies have been conducted to investigate the potential use of carotenoids in managing obesity. However, many of these studies used a combination of carotenoids and vitamins in natural sources, such as fruit juices or plant extracts, making it difficult to determine the specific effects of carotenoids alone [
164,
165,
166]. According to our knowledge, only two clinical trials have been conducted that were randomized, double-blind, placebo-controlled, and investigated the effect of pure carotenoid or xanthophyll supplementation. Canas et al. [
167] found that children who were given a mixture of carotenoids (including β-carotene, α-carotene, lutein, zeaxanthin, lycopene, astaxanthin, and γ-tocopherol) for 6 months experienced a decrease in their BMI
z-score, waist-to-height ratio, and subcutaneous adipose tissue. These positive effects were closely linked to an increase in the concentration of β-carotene in the plasma of children [
166]. Another study used a combination of paprika xanthophylls and carotenoids, given to healthy overweight volunteers for 12 weeks. This supplementation resulted in a reduction in the visceral fat area, subcutaneous fat area, and total fat area, as well as the BMI in the group that received the treatment compared to a placebo group [
166,
167,
168].