Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
While mitochondrial biogenesis has been previously demonstrated in number of differentiation models, it is only recently that the role of mitochondrial dynamics has started to be explored. The discovery of asymmetric distribution of mitochondria in stem cell progeny has strengthened the interest in the field.
- stem cell
- differentiation
- asymmetric
- mitochondria
- metabolism
1. Stemness and Cell Differentiation Are Connected to Mitochondrial Dynamics and Maintenance
Three main types of evidence suggest that stem cell maintenance and division, together with the cell fate of stem cell progeny is closely connected to mitochondrial dynamics and maintenance.
First, the mitochondrial network in stem cells is rather fragmented, while mitochondria in differentiated progeny cell display a dense tubular aspect. This difference suggests that mitochondrial fusion/fission, or more broadly mitochondrial dynamism, is needed and is essential for stemness [30].
Second, several studies show that modulation of mitochondrial dynamics and maintenance in these cells results in major consequences (reviewed in [31]). For instance, the inhibition and the knockdown of the dynamin-related protein 1 (Drp1), the main actor of the mitochondrial fission process, further differentiate iPSCs in cardiomyocytes exhibiting an oxidative metabolic shift and thus implying loss of stemness [32]. On the contrary, forced Drp1 expression promotes iPSC stemness properties [33]. Many other publications report changes in stem cell destiny and stemness properties when the actors of the mitochondrial fusion and fission mechanism (e.g., Drp1, Fis1 or OPA1) are over- or under-expressed [34,35,36,37,38,39].
Third, a variety of data support the importance of autophagy during the differentiation process. Indeed, actively oxidizing mitochondria in stem cells are removed by the mitophagy pathway, probably to preserve stemness [40]. A role for mitophagy in stemness conservation is further supported by the observation that mitophagy deficiencies promote differentiation by leaving mitochondria using primarily oxidative respiration [41]. Differentiation into the myeloid progenitor of HSCs is observed when autophagy is inhibited in these cells. Indeed, the increase in metabolically active mitochondria leads to the metabolic shift characteristic of differentiation [41,42]. Moreover, treatment of bone marrow mesenchymal stem cells (BM-MSCs) with chloroquine and 3-methyladenine, two autophagy inhibitors, results in the inhibition of BM-MSC differentiation [43]. In addition, the impairment of the PINK1–Parkin mitophagy through PINK1 deletion reduces the reprogramming efficiency of somatic cells into iPSCs, further supporting a role of the mitophagy in the stemness maintenance [34].
While it is largely accepted that mitochondria, and more particularly mitochondrial dynamics, play a prominent role in stem cell behavior, it is only recently that a potential contribution of asymmetric apportioning of mitochondria emerged as a new player in stem cell fate determination.
2. Asymmetric Mitochondrial Distribution and Stem Cell Fate
Asymmetric distribution of mitochondria in daughter cells following cell division is a phenomenon that has been described in a variety of models, ranging from yeast (reviewed in [44]) to mammalian cells. For instance, an asymmetric distribution of mitochondria is observed in the production of mouse oocytes, ensuring a high content of mitochondria in the early mammalian development and proper mitochondrial maternal inheritance [45].
In HSCs, an asymmetric distribution of organelles (mainly lysosomes and mitochondria) upon cell division has been associated with distinct cell fate, with the asymmetric distribution of mitochondria correlating with the energetic and metabolic profiles of the progenitor cells [46]. The mitochondrial network dynamic seems to play a major role to ensure this asymmetric distribution in the daughter cells which plays a central role in the stemness maintenance of HSCs. Indeed, upon fission disruption (DRP1 inhibition for instance), the HSCs lose their regenerative capacities and are blocked in a quiescent deregulated state [47]. When inducing the clearance of mitochondria, using the NAD+-boosting agent nicotinamide riboside (NR), researchers have been able to increase HSCs asymmetric divisions and to enhance their stem cell potential in a mouse model [48].
Similarly, the fusion competency of the mitochondrial network is also of utmost importance for the control of mammary stem cell differentiation. Indeed, in a model of epithelial-to-mesenchymal transition in mammary stem cell asymmetric division, the segregation of fused mitochondria close to the cortical membrane ensures the asymmetric distribution of mitochondria in the stem cell progeny. This process ensures the appropriate luminal differentiation of the progeny and the maintenance of the cortical mammary stem cell, while the disruption of the mitochondrial network fused state leads to a symmetric cell division of the progenies undergoing both luminal differentiation [49]. In addition, the proteomic analysis of the basal/cortical and luminal progenitors in mammary epithelial cells reveals a heterogeneous metabolic profile and mitochondria content [50]. Altogether, these studies further support the physiological importance of the mitochondrial asymmetric repartition leading to specific metabolic signature in stem cell progeny, ensuring tissue homeostasis through stemness and differentiation regulation.
Most interestingly, Katajisto and co-workers not only described an asymmetric distribution of mitochondria in human mammary stem cell (hMaSC) progeny but also demonstrated an asymmetry in the age of the distributed mitochondria [17]. For this experiment, the authors used a sequential Snap-tag labeling method. Briefly, the mitochondrial outer membrane protein 25 (Omp25) was first labeled with red fluorescence, and then, after a period of time, the newly produced mitochondria were labeled with green fluorescence, enabling the authors to distinguish “old” mitochondria from “young” mitochondria. Using fluorescence-activated cell sorting (FACS), they were able to sort cells according to their old or young mitochondria content. The authors showed that the cells inheriting a mixture of old and young mitochondria took the path of the differentiation, whereas the cells that retained their stemness properties received almost exclusively young mitochondria [16]. This finding suggests a link between asymmetric distribution of mitochondria based on age and cell fate determination.
Further supporting these results, Adams et al. (2016) reported similar observations in a different cell type, namely T lymphocytes, and in an in vivo setting [39]. In their study, mice infected with Listeria exhibited an asymmetric distribution of old and young mitochondria correlated with the cell fate in differentiated versus self-renewing lymphocytes. The differentiated cells showed an enrichment of old mitochondria compared to self-renewing T lymphocytes. The authors demonstrated that this asymmetric mitochondrial distribution modulated cellular metabolism, specifically the balance between catabolism and anabolism, with implications for overall cellular metabolism as explained below. The in vivo part of this study provides evidence that the age-related mitochondrial asymmetric distribution is a physiological phenomenon associated with cell differentiation and not an epiphenomenon linked to in vitro culture [39].
More recently, in 2022, the group of Katajisto pursued the characterization of the so-called “old” and “new” mitochondria and showed that the youngest mitochondria have a typical stem cell metabolism, with few oxidative phosphorylation (OxPhos) activities, low reactive oxygen species (ROS) production, and immature mitochondrial morphology (poorly developed, primitive cristae). Old mitochondria are characterized by a higher level in the electron transport chain (ETC) subunit Rieske iron-sulfur protein (RISP), responsible for the first electron transfer of the complex III. Moreover, a difference in the mitochondrial membrane potential between old and new mitochondria was observed, confirming the difference in OxPhos efficiency [17] (Figure 1). In this comprehensive review, the terms “old” and “new” are consistently employed to describe older, mature, and active mitochondria and younger, less active mitochondria, respectively, in accordance with the existing literature.
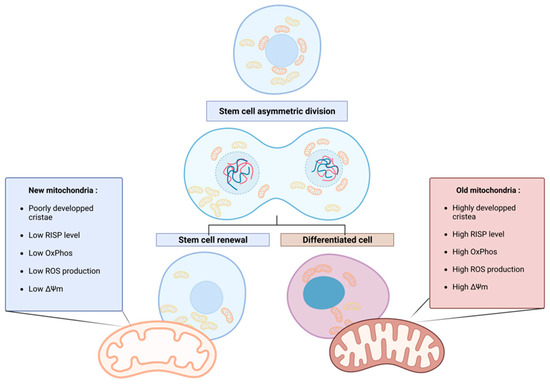
Figure 1. Asymmetric apportioning in human mammary stem-like cells (hMaSCs) and its impact on stem cell fate progeny. Old mitochondria (right) are characterized by a higher level of RISP in the ETC than young mitochondria (left), resulting in the increased oxidative metabolism of the old mitochondria, reflected in higher membrane potential (ΔΨ) and ROS levels. These characteristics initiate the differentiation of the cell inheriting old mitochondria. On the contrary, in cells receiving young mitochondria, the low oxidative metabolism maintains stem cell properties through enhanced glycolytic metabolism. Figure created with BioRender.com.
This work unveils a potential new layer of regulation for mitochondrial partitioning in the control of stemness. While the metabolic and molecular implications of the asymmetric distribution are discussed in the fifth section, the involvement of mitochondrial dynamics and quality control in the mitochondria asymmetric division are discussed below.
3. The Involvement of Mitochondrial Dynamics in Asymmetric Mitochondrial Apportioning
While the mitochondrial segregation reported during lymphocyte differentiation was observed only from the cytokinesis stage onward [39], in the model of mammary stem cells, old and young mitochondria were segregated in the cytoplasm of the mother cell before cell division, with the old ones being perinuclear and the young ones evenly distributed in the cell (Figure 1) [17]. Interestingly, the number of cells inheriting mainly young mitochondria decreases following inhibition of mitochondrial fission by a Drp1 inhibitor [16]. These results suggest that mitochondrial segregation in the mother cell is not only responsible for and leads to asymmetric partitioning but also that this mechanism is dependent on the fission, fusion, and mitophagy machinery (Drp1-dependent).
The impact of mitochondrial dynamics and activity on cell fate has been demonstrated in a recent study [49] focusing on mammary gland human stem cells, both in vitro and in vivo using mice models. The study revealed a molecular mechanism underlying the establishment of mitochondrial segregation in the mother cell during asymmetric division. In this research on a model of epithelial-to-mesenchymal transition in mammary stem cells, an asymmetric division occurs, where the fused mitochondrial network, which is more oxidative, was specifically segregated in the mother cells and subsequently polarized in the differentiated daughter cells. Of note, this segregation of fused mitochondria did not occur during symmetrical division. It is proposed that the fused mitochondria segregation in the mother cell involves a molecular mechanism that includes two key components: mitofusin 1 (MFN1), involved in the mitochondrial fusion process, and the cell polarity complex, consisting of atypical protein kinase C (aPKC), comprising PKCζ and PKCι/λ.
Activation of aPKC by TGFβ1 promotes self-renewal of stem cells and prevents the membrane localization of NUMB, a differentiation marker. Interestingly, the article reports that TGFβ1 treatment leads to membrane relocalization of both MFN1 and fused mitochondria, which is dependent on PKCζ. Indeed, those three actors (MFN1, PKCζ, and NUMB) interact, as demonstrated by co-immunoprecipitation, and the absence of PKCζ results in a shift towards symmetric cell division and a cytoplasmic localization of MFN1-labeled fused mitochondria. The proposed mechanism suggests that TGFβ1 activation leads to the relocalization of fused mitochondria to the cortical membrane, where the PKCζ-MFN1 complex anchors them. Then, the presence of MFN1 close to the membrane and in interaction with the PKCζ would be crucial for PKCζ-mediated phosphorylation of NUMB triggering its dissociation from the cortical membrane, thereby maintaining cells in a stem state [49]. Interestingly, a similar mitochondrial-to-membrane tethering mechanism is present in yeast, involving different molecular actors [44]. This supports a potentially conserved mechanism leading to mitochondrial segregation.
While the mechanisms underlying the asymmetric apportioning of young and old mitochondria in the progeny are still unclear, they seem to be not driven by the mitochondrial membrane potential (ΔΨm). Indeed, the use of a mitochondrial uncoupler did not impact the asymmetric distribution of mitochondria in hMaSC daughter cells but did affect differentiation capacity [16]. The importance of ΔΨm was revealed later in 2022 by the same group, through the increased RISP levels in old mitochondria and decreased RISP abundance in young ones. These results show that ΔΨm, and thus mitochondrial activity, is a driver of differentiation, but is not the source of the skewed distribution [17].
Beside the fusion/fission machinery, mitophagy has emerged as a major contributor to mitochondria asymmetric apportioning in stem cell division. Indeed, in addition to its well-known role in organelle homeostasis and in the response to cellular stress by the PINK1/Parkin pathway (see [51]), mitophagy contributes to determine the type of mitochondria found in a stem cell and a differentiated cell [16,39,52,53]. Both Katajisto and Adams’s studies found a higher mitophagy activity in stem-like cells (SLCs) than in epithelial cells [16] and in T/B cells compared to differentiated resident cells [39], supporting the relevance of mitochondrial clearance for self-renewal capacity. Of note, aged mitochondria colocalized with lysosomes and autophagosomes. Upon treatment with mDivi-1, an inhibitor of DRP1 protein, or with chloroquine, a general inhibitor of macroautophagy, an increase in aged mitochondria in T and B lymphocyte cells is reported, promoting the differentiation of those cells [39]. These results strongly suggest that the phenomenon of asymmetric mitochondrial distribution plays a critical role in directing cell fate, rather than being a mere consequence of the differentiation process. However, as manipulating the actors of mitochondrial dynamics also influences differentiation processes independently of asymmetric mitochondrial apportioning or even of asymmetric division (reviewed in [30]), direct evidence for a driving role of asymmetric mitochondria apportioning in cell fate decision is currently still missing.
Mitochondrial dynamics, which determines whether old mitochondria are degraded or retained, has significant implications for cellular metabolism. Stem cells degrade more their old mitochondria and thus tend to adopt a catabolic metabolism through macro autophagy while differentiating cells that retain their old mitochondria exhibit an anabolic metabolism [39]. The influence of this asymmetric distribution on cellular metabolism, in general, is the focus of investigation in the upcoming section. The objective is to understand the causal relationship between mitochondrial dynamics, including asymmetrical distribution, and cellular metabolic processes, shedding light on the broader impact of mitochondrial dynamics on cellular physiology and function.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241512181
This entry is offline, you can click here to edit this entry!
