Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hand hygiene is a crucial measure in the prevention and control of infections, and there is a growing awareness among individuals who are making a conscious effort to maintain hand cleanliness. With the advent of the SARS-CoV-2 outbreak, the demand for hand hygiene products has also gradually shifted towards those with antimicrobial properties. Among these products, hand sanitizer gels (HSGs) have gained considerable popularity as an efficient method of hand cleaning, due to their rapid drying and sustained antimicrobial efficacy.
- hand sanitizer gels
- antimicrobial properties
- biological functions
1. Introduction
Hand hygiene plays a crucial role in preventing infections and controlling the spread of diseases, as hands serve as a major medium for the transmission of pathogenic microorganisms [1,2]. Notably, over half of respiratory viruses and enterovirus infections, along with bacterial infections, are transmitted through hand-to-mouth contact among people living in close contact [3,4,5]. Therefore, hand hygiene is crucial in preventing infections and controlling the spread of epidemics, which can ultimately safeguard human life and health [6,7]. In response to the global COVID-19 pandemic, the demand for hand cleaning products with antimicrobial properties has surged [8,9]. Instant hand sanitizers (IHSs) have gained significant popularity due to their effectiveness in reducing hand pathogens, along with their convenience, accessibility, and wide consumer acceptance [10,11]. Compared to traditional soaps and wipes, IHSs are considered more convenient and efficient in reducing bacterial counts [12]. Consequently, using IHSs as an alternative to disposable wipes before meals is considered more convenient and efficient [13]. As the importance of hand hygiene continues to be emphasized, promoting the use of IHSs as an integral part of everyday routines can significantly contribute to reducing the transmission of infectious diseases and improving overall public health.
IHSs typically contain active functional ingredients, as well as inactive carrier ingredients, such as excipients, humectants, fragrances, and colorants [14,15]. They are available in various forms, including gels, liquids, sprays, and foams [16]. Each formulation has its own characteristics and considerations: (I) The low viscosity of the liquid form makes it more challenging to use effectively [17]; (II) The spray formulation of IHSs offers broader coverage, allowing for convenient application to larger surfaces. However, it requires a valve mechanism and poses a safety risk due to the flammability of ethanol. Extra precautions must be taken to avoid accidents and ensure proper storage [18]; (III) The high humidity of the foam formulation can reduce its antimicrobial properties and prolong the drying time, leading to discomfort [19]. In comparison, hand sanitizer gels (HSGs) are a popular choice due to their rapid drying time and continuous antimicrobial efficacy [20]. Additionally, HSGs form a protective layer at the application site, providing a longer period of protection on the skin. It is essential to rub HSGs thoroughly to ensure complete coverage of the hand skin (see Table 1) [21,22]. It is important to consider factors such as ease of use, safety, antimicrobial efficacy, and user comfort when selecting the most suitable IHSs for personal hygiene practices. This review will take HSGs as the main character to introduce their research progress in the field of hand hygiene.
Table 1. Different formulations of IHS and feature comparison.
| The Formulations of IHS | Inactive Ingredients | Characteristics | Ref. |
|---|---|---|---|
| Liquid formulations | Humectant, fragrance, colorant. | Widely available, but with low viscosity and hard to dispense. | [17] |
| Spray formulations | Valve actuation, humectant, fragrance, colorant. | Higher flammability risk at room temperature. | [18] |
| Foam formulations | Foaming agent, humectant, fragrance, colorant. | Longer drying time, difficult to eliminate the feeling of dissimilarity and more expensive than gel. | [19,23] |
| Gel formulations | Emollients, thickeners, neutralizers, chelators, fragrances, and dyes or colorants. | With better antimicrobial action and fast drying time, formation of a protective layer on the application site. |
[20,22] |
Since the onset of the pandemic, many countries have emphasized the implementation of non-pharmaceutical preventive measures, prominently including the intensive use of HSGs [24]. Additionally, the critical role of hand sanitizers in reducing the transmission of infectious diseases such as COVID-19 has led to a surge in global demand for HSGs since 2020, resulting in a remarkable 600-fold increase in market production [25,26]. Consequently, there has been a significant decrease in the incidence of infectious diseases in recent times [27]. This surge in market sales of HSGs has spurred the refinement of their applications, resulting in the development of a wide range of novel products with specific biological functions. These products have been designed to meet the varying needs of different consumer groups.
2. Application Scenarios for HSGs
Hand hygiene products that are widely accepted for use should have good sensory characteristics, which include a pH value similar to that of the skin, as well as appropriate viscosity and spreadability [22], as depicted in Figure 1a. Additionally, it is important not to develop an allergic reaction to the skin. The majority of hand hygiene products contain high levels of alcohol, which can cause skin irritation and dryness, as well as harm the environment [28]. Therefore, it is essential to find hand hygiene products that are safe and environmentally friendly. Products with poor sensory characteristics can reduce the frequency with which it is necessary to wash your hands [29]. The components of HSGs are biocompatible with each other at an excellent level, and the most abundant component is the thickening agent, mainly using carbomer, which is extensively used for skin and eye wound healing [30,31]. Simultaneously, carbomer has high viscosity at low concentrations and has the advantages of a wide viscosity range, great flowability, compatibility with many active ingredients, high transparency, good thermal stability, and high consumer acceptance [32,33].
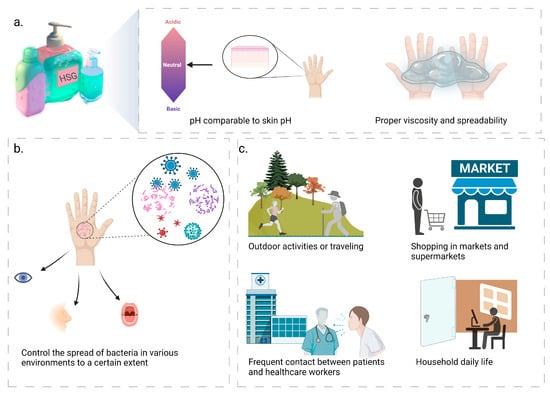
Figure 1. Hand sanitizer gels (HSGs) possess favorable sensory characteristics, exert a certain degree of control over bacterial transmission, and can be applied in various scenarios. (a) Hand hygiene products with good sensory properties, including pH similar to skin, as well as appropriate viscosity and spreadability; (b) HSGs can control the spread of bacteria in various environments to a certain extent and significantly reduce the transfer of microorganisms, thus effectively reducing respiratory and gastrointestinal infections; (c) The versatility of HSGs extends to multiple scenarios, embracing outdoor activities, supermarket shopping, health centers, domestic routines and other scenarios.
HSGs can be utilized in a wide variety of situations, owing to its effectiveness in minimizing respiratory and gastrointestinal infections based on its ability to control to a certain extent the spread of bacteria in a range of environments and to significantly reduce the transfer of microorganisms [34,35,36], as illustrated in Figure 1b,c. (I) When engaging in outdoor activities or traveling, the compact size of the HSGs allows for convenient and hygienic hand cleaning without the necessity of additional water sources and towels, thus avoiding infections and contagious diseases caused by the presence of pollutants and bacteria [37], making HSGs suitable for travel, camping, and other activities [10]; (II) during shopping in markets and supermarkets, customers frequently touch surfaces such as cart handles and shelves, resulting in bacterial contamination and transmission through hand-to-mouth and other hand-to-face touching behaviors [37,38]. In pursuit of creating a safe, healthy, and hygienic environment, HSGs offer a convenient alternative to hand soaps and other cleaning methods. They effectively eliminate hand bacteria, ensuring personal cleanliness [39]; (III) frequent contact between patients and healthcare workers can lead to the spread of bacteria in health centers such as hospitals, clinics, and pharmacies, where HSGs are more effective, act faster and can be made available at the point of patient care [40]. Studies have also shown that HSGs are generally better tolerated by the skin than soap and water [41]; (IV) family members use mobile phones, keyboards and other electronic devices daily. It is possible to spread bacteria to the hands by touching these devices [42]. In addition to furniture, doorknobs, and other common items, family members can also spread bacteria from one item to another. Therefore, using HSGs is a quick, efficient, and convenient way to help maintain good hand hygiene and prevent the spread of bacteria [34,43].
3. Classification of HSGs
HSGs can be classified into two categories based on their active ingredients: Alcohol-based HSGs (ABHSGs) and Non-alcohol-based HSGs (NABHSGs). Both types are effective in inhibiting microorganisms on the hands and no significant difference in efficacy has been observed between them [44]. Nevertheless, the mechanisms of action for these two HSGs types differ [14].
4. Application Challenges of HSGs
4.1. Antimicrobial Functional Ingredients
Since the onset of the COVID-19 pandemic, there has been a significant surge in sales of ABHSGs [70]. To meet the challenge of increased demand and raw material shortages, many countries have permitted manufacturers and medical institutions to develop and produce hand sanitizers independently. Unfortunately, this has led to a situation where some manufacturers fail to adhere to proper quality control measures or use denatured alcohol, resulting in a notable increase in substandard hand sanitizer products in the market [47,71,72,73]. Furthermore, the fermentation and distillation processes employed by manufacturers, along with the impact of production equipment and the environment, may introduce impurities such as benzene and acetaldehyde into hand sanitizers, exacerbating public health concerns [74,75]. Consequently, it is recommended that users critically evaluate the antimicrobial functional ingredients in hand sanitizer formulas and carefully review product labels to avoid potential allergens based on their personal sensitivities before use [76], as shown in Figure 2.
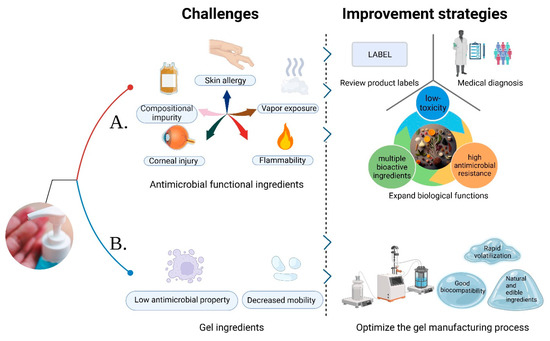
Figure 2. Application challenges and the corresponding improvement strategies of hand sanitizer gels (HSGs). (A) The challenges of antimicrobial functional ingredients at present and the corresponding improvement measures are put forward; (B) The current challenges associated with gel ingredients are identified, along with proposed measures for their improvement.
Common antimicrobial components found in regular hand sanitizer products (such as ABHSGs) can cause corneal epithelial cell destruction at high concentrations, leading to eye diseases. Prolonged contact with hands may also increase the risk of local adverse reactions, such as contact dermatitis and atopic dermatitis [77]. In recent years, clinical cases involving adverse effects from ABHSGs have been on the rise, particularly among children [78,79]. Children tend to use hand sanitizers more frequently than adults [80], and face an increased risk of ingestion and exposure problems after using informal ABHSGs, underscoring the importance of adult supervision during use [78,81]. Additionally, healthcare workers who apply ABHSGs before handling newborns may heighten the risk of neonatal exposure to ethanol vapor, potentially causing neuronal damage and leading to neurodevelopmental delay and behavioral issues. However, the impact of such low-dose ethanol exposure on neonatal brain development is currently unclear [82,83]. Furthermore, isopropanol ingestion can result in common health problems. Accidental inhalation or dermal exposure may cause poisoning, although it usually does not lead to serious health hazards [84]. Repeated exposure of microbes to disinfectants, antibiotics, and other genotoxic chemicals leads to the development of resistance, becoming a significant global concern, particularly burdening healthcare professionals [85]. Triclosan serves as a prime example, having been used as an antimicrobial component in NABHSGs over the past decades, but evidence indicates its potential for environmental impact and antibiotic resistance [86]. Therefore, excessive and prolonged use of HSGs also entails potential risks. Furthermore, the impact of ethanol, isopropanol, and other ingredients on the environment has long been a topic of concern [87]. These compounds can volatilize or seep into soil and groundwater, significantly affecting aquatic organisms [88]. Thus, while addressing potential issues associated with the long-term use of HSGs, it is crucial to emphasize effective management of good hygiene practices.
To maintain hand hygiene, particularly among children and healthcare workers, frequent hand washing is crucial. Therefore, it is important to select an emollient that effectively strengthens the skin barrier. Research indicates that BAK, an antiseptic ingredient, provides immediate and long-term antimicrobial effects, and the addition of emollients typically does not diminish its effectiveness [89]. Furthermore, BAK tends to be less irritating to the skin and rarely causes allergic reactions [63]. In cases where adverse reactions, such as hand rashes, arise from using ABHSGs, it is recommended to either switch to hand sanitizers containing BAK or seek medical treatment [90]. It is important to note that ethanol and isopropanol, commonly found in ABHSGs, are volatile and combustible substances that could cause fires when used near flames or exposed to high temperatures [16,18,91]. As such, the inclusion of novel safe antimicrobial ingredients can offer a safer alternative with an improved safety profile.
4.2. Gel Ingredients
The stability of the gel is related to the pH of the compound, with a lower pH resulting in decreased mobility. Considering that ethanol and isopropanol have different polarities, and isopropanol exhibits a significantly lower polarity compared to ethanol, a greater amount of triethanolamine must be incorporated into isopropanol to produce a stable polymer with carbomer [92]. Studies have demonstrated that anionic thickeners (such as carbomer or acrylate) notably impair the antimicrobial persistence of ABHSGs on the skin. As an alternative, non-ionic polymer thickeners like hydroxypropyl cellulose may be considered to replace carbomer and improve antimicrobial persistence [93]. In addition, by optimizing the manufacturing process of the gel, it is possible to explore materials with good biocompatibility, fast volatilization, and even completely natural edible ingredients in HSGs, which not only contribute to the stability of the gel, but also provide enhanced biocompatibility, leading to faster volatilization. This exploration opens up the possibility of reducing reliance on synthetic ingredients and instead adopting entirely natural and edible ingredients. The trend towards using natural and edible ingredients in hand hygiene products is in line with consumers’ growing preference for environmentally friendly and safe options.
This entry is adapted from the peer-reviewed paper 10.3390/toxics11080687
This entry is offline, you can click here to edit this entry!
