Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
High-grade gliomas are malignant brain tumors, and patient outcomes remain dismal despite the emergence of immunotherapies aimed at promoting tumor elimination by the immune system. A robust antitumor immune response requires the presentation of tumor antigens by dendritic cells (DC) to prime cytolytic T cells.
- dendritic cells
- glioma
1. Introduction
High-grade gliomas (HGG) refer to a set of highly aggressive and lethal brain tumors that arise from glial cells. HGG are the most frequent primary CNS malignancy and include astrocytoma, oligodendroglioma, and glioblastoma (GBM), as classified by the updated World Health Organization guidelines [1]. The current standard of care leaves patients with a median survival time of only fifteen months and a five-year survival rate of merely 6.8%, indicative of an urgent clinical need for improved treatment regimens [2][3]. The standard treatment regimen consists of temozolomide chemotherapy, radiotherapy, and tumor resection [2]. Although this combination can acutely limit tumor progression, HGG are diffuse throughout the brain and inevitably recur as a result of malignant cells that remain in small numbers [4][5]. Standard treatment for HGG has not changed in nearly two decades, despite advances in researchers' understanding of cancers, neuroimmunology, and therapeutic options.
Immunotherapies are one of the promising options available, given that they can leverage the patient’s immune system to recognize and eliminate HGG cells. Although the diffuse sections of HGG are challenging to isolate for treatment with radiotherapy or resection, immune cells have the potential to target them more specifically. Effective immunotherapies can also induce long-term immune memory, which may prevent or slow the recurrence that currently plagues HGG patients. Despite success in treating other types of cancer, clinical trials using inhibitor checkpoint blockade (ICB), vaccination strategies, and CAR T cells have yet to show substantial benefit in HGG patients. However, relatively little is known about the immunological landscape of HGG, which constrains researchers' ability to make advances with immunotherapy for these diseases compared to other more commonly and deeply studied extracranial tumors.
Fortunately, the field of neuroimmunology is quickly growing, and advancements in basic research will be critical for the identification and optimization of impactful immunotherapeutic regimens. This is especially important considering patients whose tumors fail to respond to immunotherapies and the potentially toxic side effects that accompany their delivery. Antigen presentating cells (APC), specifically dendritic cells (DC), are of particular interest due to their role in initiating and sustaining the T cell responses that are required for substantive and durable tumor control.
2. Dendritic Cells Function in Antitumor Responses
The innate and adaptive arms of immunity must function cohesively to coordinate a robust immune response. For this reason, DC are critical mediators of immunity. In the fifty years since DC were first described by Ralph Steinman, many DC subsets have been observed to infiltrate a wide variety of tumors, albeit in relatively low proportions [6]. Although the definitions of these DC subsets vary with time and the method used for identification, apart from plasmacytoid DC (pDC) they generally share the ability to induce adaptive immune responses from naïve T cell precursor populations. Conventional DC (cDC) accomplish this by providing essential context beyond TCR engagement, largely through a combination of costimulatory molecule expression and cytokine secretion.
DC are classic professional antigen presenting cells (pAPC) that are either poised in the tissue to phagocytose antigens and migrate to draining lymph nodes or are resident in the lymph nodes where they capture cell-free antigens from lymphatic drainage. DC process acquired antigens for presentation on MHC class I molecules for presentation to CD8+ T cells, and MHC class II molecules for presentation to CD4+ T cells. In addition to this fundamental role in stimulating MHC-peptide specific T cells, when appropriately activated, DC provide critical costimulatory signals that are necessary to promote T cell cycling and expansion, rather than the induction of anergic unresponsiveness.
A heterogeneous population, DC are further defined into four subsets, each with unique characteristics. cDC1 (defined by XCR1 and DNGR-1 expression) are essential for many antitumor immune responses due to their ability to cross-present tumor antigen to cytotoxic CD8+ T cells and to secrete significant amounts of IL-12. Cross-presentation serves as a critical link for priming CD8+ T cells, as most cells that express the MHC class I-ligand that is recognized by CD8+ T cells do not express the critical costimulatory molecules necessary for robust CD8+ T cell activation and expansion. This makes the role of cDC1 especially relevant to antitumor responses since they can uniquely redirect exogenous, phagocytosis-acquired antigens to this pathway for presentation to CD8+ T cells [7][8]. cDC1 also contribute to the antitumor response through antigen presentation to CD4+ T cells, resulting in the induction of CD40L on T cells, which leads to cDC1 licensing and a subsequently enhanced CD8+ T cell response [9]. Moreover, cDC1 are the primary producers of IL-12, a cytokine secreted during antigen presentation that induces Tbet, a transcription factor that empowers effector differentiation in CD8+ T cells and polarizes CD4+ T cells toward a Th1 phenotype with enhanced IFN-γ production and proliferative capacity [10].
A second subset, cDC2 (commonly, though not perfectly, defined by CD11b and SIRPα expression), also contribute to the antitumor response, primarily through antigen presentation to CD4+ T cells via MHCII [11]. Unlike cDC1, the precise role of cDC2 in anti-tumor T cell responses has been more challenging to define, since deletion of single genes involved in cDC2 differentiation does not thoroughly ablate cDC2 in mouse models [12]. Differentiated from infiltrating monocytes under inflammatory conditions, monocyte-derived DC (moDC) appear to overlap with cDC in function yet do not have the same cross-presentation capacity as cDC1 [9]. pDC represent a fourth subset of DC that uniquely differentiate from lymphoid rather than myeloid precursors [13]. Generally dispensable for antigen presentation and T cell priming, pDC are better known for secreting large quantities of Type-1 IFN upon TLR stimulation, which can support cDC-mediated initiation of primary T cell responses. However, tumor infiltrating pDC do not exhibit this function, likely a result of the immunosuppressive factors in the TME [14]. Instead, tumor-infiltrating pDC can recruit Tregs, a highly immunosuppressive subset of CD4+ T cells [15]. Overall, DC represent a heterogeneous population of cells with both immunogenic and tolerizing potential within the TME and make logical immunotherapeutic targets due to their ability to promote antitumor immunity, as described in more detail below.
In addition to the subtypes of DC, the character of their interaction with T cells can determine the type of immune response that ensues. DC can be immunogenic or tolerogenic depending on the milieu of their environment and maturation status. Upon priming in the presence of IL-12, which is largely expressed by DC, activated naive T cells differentiate into Th1 cells that produce IFN-γ, a cytokine critical for imposing an inflammatory cytokine signaling loop [16]. In the absence of stimulation via PRRs or cytokines such as Type 1 IFN, DC may still phagocytose and present antigens to T cells; however, these DC do not upregulate expression of MHC or costimulatory molecules. This can lead to T cell anergy and tolerization to the antigen presented [17][18]. Immature DC are also more likely to be tolerogenic, likely due to underdeveloped antigen processing pathways or low level of MHC and costimulatory molecules after antigen uptake; however, mature DC can also be tolerogenic depending on the stimulus [19][20]. Within the context of tumors, it is established that DC tend to have reduced antigen presentation capacity and an immature phenotype [21][22][23]. The HGG TME is characteristically immunosuppressive, with high levels of TGF-B, VEGF, prostaglandins, and IL-10—all factors that can inhibit DC activity as illustrated in Figure 1 [24][25][26][27][28][29][30].
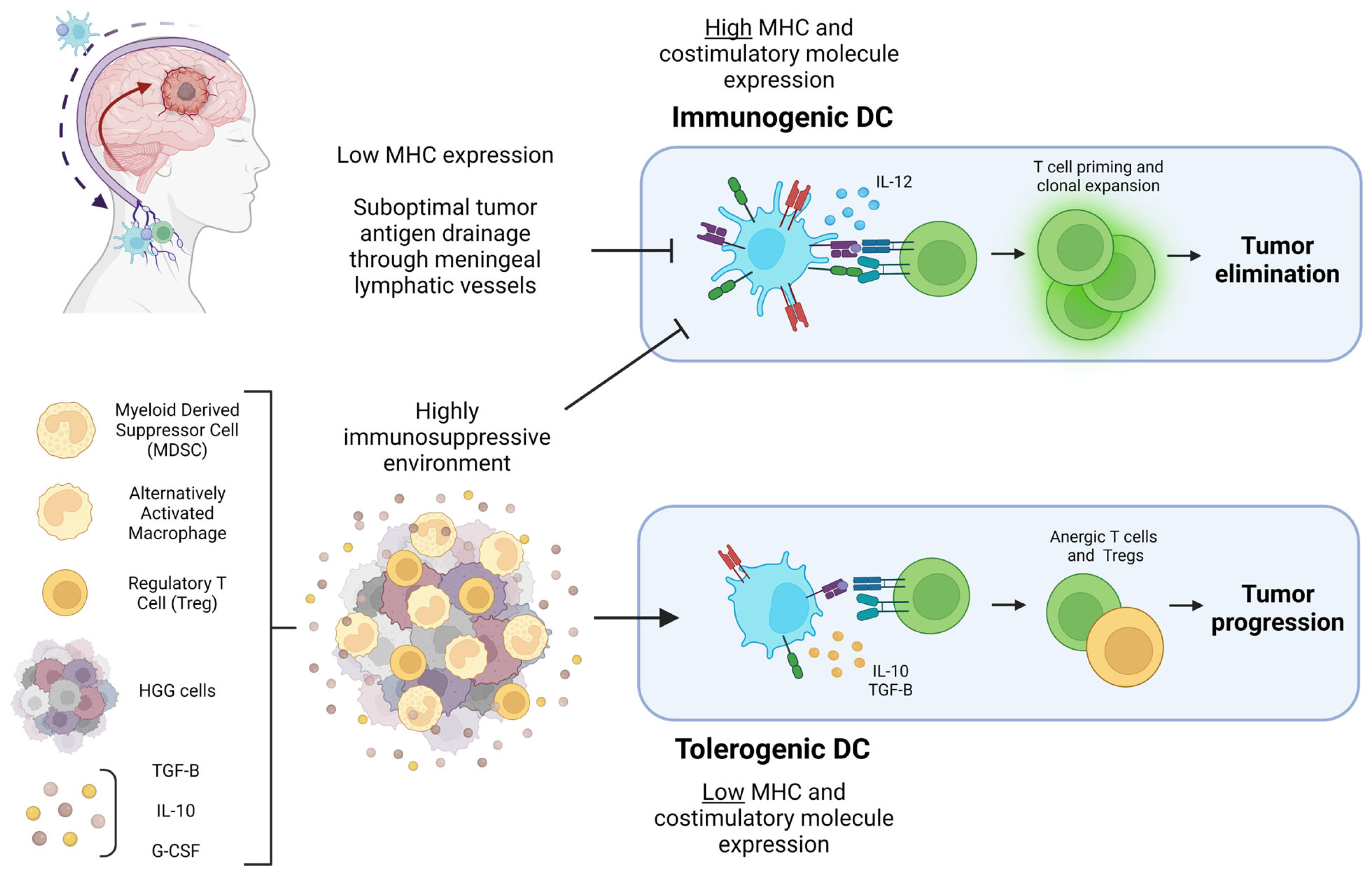
Figure 1. Diagram of the HGG-associated factors that can lead to tumor elimination or progression through DC polarization. Pictured in the top left corner is a diagram of HGG antigen drainage through meningeal lymphatic vessels to draining cervical lymph nodes mediated by DC. Suboptimal tumor antigen drainage prevents T cell priming and clonal expansion in the cervical lymph nodes. The highly immunosuppressive HGG TME pictured at the bottom left prevents DC from expressing the costimulatory molecules necessary for immunogenic T cell priming. Cytokines and immunosuppressive cells within the TME result in DC with a tolerogenic character that ultimately prime anergic T cells and Tregs, contributing to tumor progression. Figure created with Biorender.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15112902
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro. Oncol. 2021, 23, 1231–1251.
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro. Oncol. 2019, 21, v1–v100.
- Dandy, W.E. Removal of Right Cerebral Hemisphere for Certain Tumors with Hemiplegia: Preliminary Report. J. Am. Med. Assoc. 1928, 90, 823–825.
- Putavet, D.A.; de Keizer, P.L.J. Residual Disease in Glioma Recurrence: A Dangerous Liaison with Senescence. Cancers 2021, 13, 1560.
- Steinman, R.M.; Cohn, Z.A. Identification of a Novel Cell Type in Peripheral Lymphoid Organs of Mice. I. Morphology, Quantitation, Tissue Distribution. J. Exp. Med. 1973, 137, 1142–1162.
- Bevan, M.J. Cross-Priming for a Secondary Cytotoxic Response to Minor H Antigens with H-2 Congenic Cells Which Do Not Cross-React in the Cytotoxic Assay. J. Exp. Med. 1976, 143, 1283–1288.
- den Haan, J.M.; Lehar, S.M.; Bevan, M.J. CD8(+) but Not CD8(−) Dendritic Cells Cross-Prime Cytotoxic T Cells in vivo. J. Exp. Med. 2000, 192, 1685–1696.
- Ferris, S.T.; Durai, V.; Wu, R.; Theisen, D.J.; Ward, J.P.; Bern, M.D.; Davidson, J.T.; Bagadia, P.; Liu, T.; Briseño, C.G.; et al. CDC1 Prime and Are Licensed by CD4+ T Cells to Induce Anti-Tumour Immunity. Nature 2020, 584, 624–629.
- Heufler, C.; Koch, F.; Stanzl, U.; Topar, G.; Wysocka, M.; Trinchieri, G.; Enk, A.; Steinman, R.M.; Romani, N.; Schuler, G. Interleukin-12 Is Produced by Dendritic Cells and Mediates T Helper 1 Development as Well as Interferon-γ Production by T Helper 1 Cells. Eur. J. Immunol. 1996, 26, 659–668.
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity. Cell 2019, 177, 556–571.e16.
- Anderson, D.A.; Murphy, K.M.; Briseño, C.G. Development, Diversity, and Function of Dendritic Cells in Mouse and Human. Cold Spring Harb. Perspect. Biol. 2018, 10, a028613.
- Rodrigues, P.F.; Alberti-Servera, L.; Eremin, A.; Grajales-Reyes, G.E.; Ivanek, R.; Tussiwand, R. Distinct Progenitor Lineages Contribute to the Heterogeneity of Plasmacytoid Dendritic Cells. Nat. Immunol. 2018, 19, 711–722.
- Zhou, B.; Lawrence, T.; Liang, Y. The Role of Plasmacytoid Dendritic Cells in Cancers. Front. Immunol. 2021, 12, 749190.
- Aspord, C.; Leccia, M.-T.; Charles, J.; Plumas, J. Plasmacytoid Dendritic Cells Support Melanoma Progression by Promoting Th2 and Regulatory Immunity through OX40L and ICOSL. Cancer Immunol. Res. 2013, 1, 402–415.
- Cools, N.; Ponsaerts, P.; Van Tendeloo, V.F.I.; Berneman, Z.N. Balancing between Immunity and Tolerance: An Interplay between Dendritic Cells, Regulatory T Cells, and Effector T Cells. J. Leukoc. Biol. 2007, 82, 1365–1374.
- Schwartz, R.H.; Mueller, D.L.; Jenkins, M.K.; Quill, H. T-Cell Clonal Anergy. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 605–610.
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions In Vivo. J. Exp. Med. 2001, 194, 769–779.
- Wilson, N.S.; El-Sukkari, D.; Villadangos, J.A. Dendritic Cells Constitutively Present Self Antigens in Their Immature State In Vivo and Regulate Antigen Presentation by Controlling the Rates of MHC Class II Synthesis and Endocytosis. Blood 2004, 103, 2187–2195.
- Akbari, O.; DeKruyff, R.H.; Umetsu, D.T. Pulmonary Dendritic Cells Producing IL-10 Mediate Tolerance Induced by Respiratory Exposure to Antigen. Nat. Immunol. 2001, 2, 725–731.
- Gabrilovich, D.; Corak, J.; Ciernik, I.; Kavanaugh, D.; Carbone, D. Decreased Antigen Presentation by Dendritic Cells in Patients with Breast Cancer. Clin. Cancer Res. 1997, 3, 483–490.
- Pinzon-Charry, A.; Maxwell, T.; López, J.A. Dendritic Cell Dysfunction in Cancer: A Mechanism for Immunosuppression. Immunol. Cell. Biol. 2005, 83, 451–461.
- Kusmartsev, S.; Gabrilovich, D.I. Effect of Tumor-Derived Cytokines and Growth Factors on Differentiation and Immune Suppressive Features of Myeloid Cells in Cancer. Cancer Metastasis Rev. 2006, 25, 323–331.
- Hishii, M.; Nitta, T.; Ishida, H.; Ebato, M.; Kurosu, A.; Yagita, H.; Sato, K.; Okumura, K. Human Glioma-Derived Interleukin-10 Inhibits Antitumor Immune Responses In Vitro. Neurosurgery 1995, 37, 1160–1166; discussion 1166–1167.
- Ikushima, H.; Todo, T.; Ino, Y.; Takahashi, M.; Miyazawa, K.; Miyazono, K. Autocrine TGF-Beta Signaling Maintains Tumorigenicity of Glioma-Initiating Cells through Sry-Related HMG-Box Factors. Cell Stem Cell 2009, 5, 504–514.
- Turkowski, K.; Brandenburg, S.; Mueller, A.; Kremenetskaia, I.; Bungert, A.D.; Blank, A.; Felsenstein, M.; Vajkoczy, P. VEGF as a Modulator of the Innate Immune Response in Glioblastoma. Glia 2018, 66, 161–174.
- Mattila, S.; Tuominen, H.; Koivukangas, J.; Stenbäck, F. The Terminal Prostaglandin Synthases MPGES-1, MPGES-2, and CPGES Are All Overexpressed in Human Gliomas. Neuropathology 2009, 29, 156–165.
- Himes, B.T.; Geiger, P.A.; Ayasoufi, K.; Bhargav, A.G.; Brown, D.A.; Parney, I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021, 11, 770561.
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive Mechanisms in Glioblastoma. Neuro-Oncology 2015, 17, vii9–vii14.
- Zong, J.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Tumor-Derived Factors Modulating Dendritic Cell Function. Cancer Immunol. Immunother. 2016, 65, 821–833.
This entry is offline, you can click here to edit this entry!
