Positron Emission Tomography/Computed Tomography (PET/CT) has been increasingly used in bladder cancer staging to improve the accuracy of lymph node detection and to overcome the lack of sensitivity and the understaging showed by conventional imaging. Lymph node (LN) involvement is a crucial determinant of prognosis for patients with bladder cancer, and an accurate staging is of utmost importance to better identify timely and appropriate therapeutic strategies. To improve the accuracy of LN detection, as an alternative to traditional methods such as CT or MRI, 18F-FDG PET/CT has been increasingly used. 18F-FDG PET/CT is also used in post-treatment restaging after neoadjuvant chemotherapy.
1. Introduction
Bladder Cancer (BCa) is a widespread disease that is ranked as the 10th most commonly diagnosed form of cancer globally. It has a notable effect on the wellbeing and longevity of individuals affected, in particular in situations where the cancer has progressed into a muscle invasive disease
[1].
In fact, even if 70% of bladder cancer is represented by non-muscle-invasive tumors, the remaining 30% of patients have muscle-invasive bladder cancer (MIBC) associated with a high risk of lymph node (LN) involvement and distant metastases
[2][3].
The presence of LN involvement in patients with BC, which indicates that cancer has progressed beyond the organ, is a crucial determinant of prognosis, with significant implications for treatment response and overall survival. The prognosis in cases of node-positive disease depends on multiple factors, including the stage and extent of the disease, as well as the presence of other risk factors such as advanced age, performance status, and comorbidities
[4]. As such, a comprehensive evaluation of these factors is necessary to determine the optimal course of treatment and improve outcomes in affected individuals
[5]. The management of node positive bladder cancer may involve a combination of surgery, chemotherapy, and/or radiation therapy.
It is important for these patients to receive an accurate staging of the bladder tumor to better identify timely and appropriate therapeutic strategies to improve their chances of survival and their quality of life
[6]. Before a radical cystectomy, preoperative locoregional staging is important to define the indication to neoadjuvant chemotherapy (NAC) to eradicate any micro-metastatic disease or eventually to guide the clinician towards an extended pelvic LN dissection to improve the chances of performing a curative surgery. It is crucial to identify patients with advanced metastatic disease and carefully evaluate the indication for a salvage radical cystectomy
[7]. If the surgery is not curative but only palliative, doctors and patients must consider the impact of a urinary diversion on the patient’s quality of life. Therefore, it is essential to weigh the potential benefits and drawbacks of the surgery and engage in a shared decision-making process to ensure that the patient’s goals and preferences are taken into account
[8][9].
In routine clinical practice, preoperative staging of muscle-invasive bladder cancer (MIBC) typically involves performing a computed tomography (CT) scan of the chest, abdomen, and pelvis. Pelvic Magnetic Resonance Imaging (MRI) can also be used, although not routinely in clinical practice. However, despite their accuracy in detecting primary bladder disease, both CT and MRI have not proven to have high sensitivity for nodal staging. With a sensitivity of 50–85% for the detection of pelvic LN involvement, both CT and MRI understage about 1/3 of patients. CT and MRI remain valuable tools in the preoperative staging of muscle-invasive bladder cancer and can provide useful information for guiding treatment decisions. To improve the accuracy of LN detection, other imaging modalities such as positron emission tomography (PET) or sentinel LN biopsy may be considered in selected cases
[10].
To overcome this lack of sensitivity in identifying lymph node involvement, Positron Emission Tomography/Computed Tomography (PET/CT) has been increasingly used in bladder cancer staging.
PET/CT is a non-invasive imaging modality which has gained increasing popularity in the evaluation of patients with cancer and provides whole-body imaging, even if imaging of chest, abdomen, and pelvis might be enough for many cancers
[5].
PET/CT combines the functional information supplied by PET with the anatomic detail of CT, providing comprehensive information about the metabolic and structural changes in the body.
In particular, 18 F-fluoro-2-deoxy-D-glucose (18F-FDG) PET/CT confers information based on glucose uptake and identifies cells with a high uptake such as neoplastic cells with their increased utilization of glucose.
Since metabolic alterations occur before the morphological ones, 18F-FDG PET/CT enables an early detection of locoregional disease, distant metastases, and cancer recurrence, before they become evident by conventional imaging like TC or MRI.
Another important topic about MIBC is the post-treatment restaging after neoadjuvant chemotherapy.
2. Performance of 18F-FDG PET/CT in Bladder Cancer
18F-FDG PET/CT has demonstrated promising results in detecting and staging various human cancers, as evidenced by previous studies
[11]. However, the evidence regarding its use in bladder cancer is still a matter of debate. The use of 18F-FDG in primary bladder cancer detection is limited due to its high urinary excretion in the bladder and ureters which could be a confounding factor for detection of bladder wall lesions and metastatic regional LNS
[12]. Identifying peri-vesical LN can be particularly challenging as they may be too small to be detected on CT or may be masked by adjacent 18F-FDG excretion in the urinary tract on PET/CT. The urinary excretion of 18F-FDG can lead to increased background activity in the pelvis, which can make it difficult to distinguish small LNs from surrounding urinary activity. As a result, accurate detection of peri-vesical LNs may require the use of more advanced imaging techniques, such as diffusion-weighted imaging (DWI) or dynamic contrast-enhanced MRI (DCE-MRI). Nonetheless, 18F-FDG PET/CT remains a valuable tool in identifying LN involvement and guiding treatment decisions in patients with bladder cancer.
Several measures may be employed to improve the accuracy of 18F-FDG PET/CT in bladder cancer imaging. These include oral pre-hydration to dilute the urinary tracer and the use of diuretics to enhance local regional accuracy in scans following furosemide administration. Bladder catheterization may also be effective in limiting 18F-FDG accumulation in the bladder and ureters, thereby improving the detection of bladder wall lesions and regional LNs. While these measures may improve the accuracy of 18F-FDG PET/CT in bladder cancer imaging, their routine use may not be practical or feasible in all patients, and their benefits should be weighed against potential risks and inconveniences
[12][13]. To date, the use of F-18 FDG PET/TC for detection of LN metastasis in bladder cancer is controversial and uncertain: some studies showed low accuracy rates, but over the years other studies have reported evidence of high sensitivity and specificity
[5][14].
Previous studies have suggested that the advantage of combined PET/CT over CT alone in detecting bladder cancer is minimal, likely due to the significant overlap between standardized uptake values (SUVs) of malignant lesions and active inflammatory processes. This overlap in SUVs can limit the specificity of 18F-FDG PET/CT in detecting bladder tumors and regional LNs, particularly in cases where there is significant inflammation or infection in the bladder or adjacent tissues.
While the combination of PET and CT imaging may still provide some additional information beyond what can be obtained with either modality alone, the limited advantage in diagnostic accuracy suggests that the routine use of combined PET/CT for bladder cancer imaging may not be justified in all cases.
A review of 2012 showed a sensitivity for combined PET-CT scan of 82% (95% CI: 0.72–0.89)
[12].
Girard et al. in 2018 concluded that 18F-FDG PET/CT correctly detect LNs involvement in an additional 8% of patients compared to CT alone and that 18F-FDG PET/CT accuracy is 82% compared to 74% of CT alone
[10]. Several studies have also shown that combined 18F-FDG PET/CT is superior for detection of distant metastases in bladder cancer
[5][10]. The ability of PET to detect small metastases or LNs with high metabolic activity can increase the specificity of 18F-FDG PET/CT in detecting bladder cancer and regional metastasis
[15].
According to Goodfellow et al., PET scans are considered useful if they result in a change in management for more than 10% of patients. In such cases, routine use of PET scans would be recommended. When PET scans lead to a change in management in 5–10% of patients, they should be used selectively for certain patients. However, if PET scans result in a change in management for less than 5% of patients, their routine use may not be justified. These recommendations highlight the need for careful consideration of the potential benefits and limitations of PET imaging, and the importance of individualized decision-making in the management of bladder cancer
[16].
A recent review of 2022 on preoperative detection of pelvic LN involvement confirmed a higher sensitivity and specificity combining PET and CT scan when compared to the traditional imaging modalities
[5]. A recent consensus statement by the European Association of Urology (EAU) and the European Society for Medical Oncology (ESMO) stated that 18F-FDG PET/CT should be included in oligometastatic disease staging to minimize the risk of overtreatment, when radical treatment options are being considered
[15].
18F-FDG PET/CT has multiple applications beyond preclinical staging, including post-treatment restaging after neoadjuvant chemotherapy (NAC). Traditional modalities, such as cytology, cystoscopy, CT scans, MRIs, and routine blood tests, are often inaccurate in detecting residual disease or assessing treatment response. Early assessment of NAC response and/or residual disease is crucial for guiding perioperative management, limiting chemotherapy-related side effects and improving quality of life. 18F-FDG PET/CT is a reliable tool for monitoring response to chemotherapy in various cancer types and has been shown to be more accurate than conventional imaging. Recent studies have identified 18F-FDG PET/CT as an effective method for detecting both residual and recurrent disease, with superior accuracy in detecting post-treatment recurrence outside the urinary tract, primarily bone lesions, compared to conventional restaging techniques. Despite its potential benefits, there is currently no consensus on the use of 18F-FDG PET/CT for identifying chemo-sensitive bladder tumors during NAC. Nonetheless, the use of 18F-FDG PET/CT in post-treatment restaging may help to guide treatment decisions, such as whether to proceed with radical cystectomy or continue with NAC, based on the presence or absence of residual disease
[5].
In addition to its potential role in bladder cancer staging, 18F-FDG PET/CT has applications in sentinel lymph node (SLN) mapping to aid in the resection of selected, invaded LNs during pelvic LND
[17]. This approach can simplify histopathological examination and reduce the extent of LN dissection (LND) compared to blind template resection. Lymphoscintigraphy, CT, MRI, and fluoroscopy are commonly used methods for SLN mapping. SLN biopsy (SLNB) has been successfully used in breast and skin cancer treatment, contributing to a reduction in LND extent. Recent evidence suggests that 18F-FDG PET/CT combined with CT or MRI can be useful in evaluating LNs suspected to be involved based on CT/MRI findings, with a sensitivity of 92% and specificity of 91%. However, its sensitivity is considerably lower (7–23%) in patients with no suspicion of LN involvement on CT. Studies have shown that SLN mapping has a high detection rate and sensitivity in MIBC, particularly in patients with low pT stage bladder cancers and clinically negative LNs.
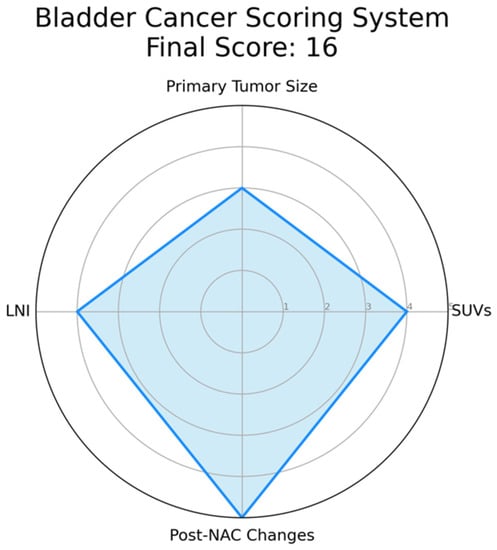
The complexity of bladder cancer staging using PET/CT prompts the need for a scoring system to improve its precision. This system should integrate key parameters like standardized uptake values (SUVs), indicative of tumor metabolic activity, and primary tumor size, which could correlate with disease progression. Lymph node involvement, particularly peri-vesical nodes, and the presence of distant metastases identified by PET/CT should also be incorporated due to their prognostic implications. Furthermore, changes in PET/CT findings post-neoadjuvant chemotherapy (NAC) could provide insights into tumor chemosensitivity and could thus influence the score. This score has been explained in detail in Figure 1.
Figure 1. Example of a Radar Chart for the Proposed Bladder Cancer Scoring System. This chart represents an illustrative application of the proposed scoring system. Each axis corresponds to a parameter of the scoring system: standardized uptake values (SUVs), primary tumor size, lymph node involvement, and post-neoadjuvant chemotherapy (NAC) changes. The value along each axis represents the score for that parameter. SUVs is assigned as follows: 1 if SUV ≤ 2.5, indicating low metabolic activity and potentially less aggressive disease; 2 if 2.5 < SUV ≤ 5.0, suggesting moderate metabolic activity; 3 if 5.0 < SUV ≤ 7.5, suggesting relatively higher metabolic activity; 4 if 7.5 < SUV ≤ 10.0, indicative of high metabolic activity; 5 if SUV > 10.0, indicative of extremely high metabolic activity and potentially more aggressive disease. Primary tumor size is defined as follows: 1 if tumor size ≤ 2 cm, considered a small tumor; 2 if 2 cm < tumor size ≤ 4 cm, considered a moderately sized tumor; 3 if 4 cm < tumor size ≤ 6 cm, considered a relatively larger tumor, 4 if 6 cm < tumor size ≤ 8 cm, considered a large tumor, 5 if tumor size > 8 cm, considered a very large tumor, often associated with a worse prognosis. Lymph Node Involvement is defined as follows: 1 in case of no lymph nodes involved; 2 in case of single lymph node involvement; 3 in case of 2–3 lymph nodes involved, 4 in case of 4–6 lymph nodes involved, 5 in case of more than 6 lymph nodes involved, indicating extensive disease spread. Post-Neoadjuvant Chemotherapy (NAC) Changes are summarized as follows: 1 in case of no reduction in tumor size post-NAC, indicating no response; 2 in case of 1–25% reduction in tumor size post-NAC, suggesting a minimal response; 3 in case of 25–50% reduction in tumor size post-NAC, indicative of a partial response; 4 in case of 50–75% reduction in tumor size post-NAC, indicating a good response; 5 in case of more than 75% reduction or complete disappearance of the tumor post-NAC, indicating an excellent response. In this instance, hypothetical values are used for demonstration purposes: SUVs (4), Tumor Size (3), Lymph Node Involvement (4), and Post-NAC Changes (5). The filled area in the chart represents the composite score profile for a patient, providing a visual summary of the tumor’s characteristics according to the scoring system. Please note that in an actual case, values would be derived from clinical and imaging data, not randomly assigned.
The development of this scoring system calls for rigorous validation through future studies, correlating the score with patient outcomes in various settings and patient groups. Such a scoring system could reconcile PET/CT discrepancies and enhance its utility in bladder cancer assessment. However, ongoing research is essential to confirm the value of this proposed scoring system, as it presents a promising avenue to optimize PET/CT’s role in bladder cancer management.
Despite its potential benefits, 18F-FDG PET/CT has several limitations, including high cost, higher radiation exposure, lack of anatomic reference frame when performed alone, and prolonged lag time between PET and staging CT scans. A full-dose diagnostic staging CT with intravenous contrast medium may provide a better assessment of LNs and metastases than the CT component of the PET scan. False positive results can also lead to delay of treatment, unnecessary procedures, and additional costs. Therefore, PET scan results should be interpreted with caution in conjunction with CT scan and clinical judgment, particularly in cases of benign tumors or inflammatory lesions. Nonetheless, accurate staging information provided by 18F-FDG PET/CT can significantly influence therapeutic management and serve as an important prognostic indicator for progression-free survival (PFS) and overall survival (OS).
In fact, an early evaluation of NAC response and of presence of residual disease is important to guide the perioperative management of patients. For example, patients with advanced disease and persistent LN involvement even after NAC have poor prognosis, and a multidisciplinary team has to evaluate whether to proceed with a radical cystectomy, intended for palliative rather than curative purpose. Patients who are unresponsive to neoadjuvant chemotherapy (NAC), particularly those with localized disease (cT2-T4aN0M0), might see more benefits from an immediate radical cystectomy rather than continuing NAC, especially considering chemotherapy-related side-effects.
Methods traditionally used for post-treatment restaging, such as cystoscopy, urine cytology, routine blood tests, CT, and MRI scans, do not have high diagnostic accuracy, and there is no consensus recommendation regarding restaging imaging during NAC.
5. Conclusions
Although 18F-FDG PET/CT has shown promise as a feasible and reliable tool for bladder cancer staging, evidence for its use in diagnosis and staging is not yet strong. Like all radiological exams, 18F-FDG PET/CT is limited by the inability to retrieve a histological sample. Its non-invasive nature also makes it a potential tool for follow-up, but further studies are needed to evaluate its effectiveness in this setting. A standardized reporting system for characterizing bladder cancer using 18F-FDG PET/CT is still lacking, and efforts should be aimed at developing and validating a scoring system to improve its accuracy in staging. Despite these limitations, 18F-FDG PET/CT remains a valuable tool in the management of bladder cancer and can provide important information for treatment planning and prognostication.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15112951