Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Alginates are polysaccharides that are produced naturally and can be isolated from brown sea algae and bacteria. Sodium alginate (SA) is utilized extensively in the field of biological soft tissue repair and regeneration owing to its low cost, high biological compatibility, and quick and moderate crosslinking.
- sodium alginate
- hydrogels
- tissue engineering
- characterization
1. Introduction
The majority of human organs are made up of soft tissues, and since these tissues can regenerate, patients will have a far lower chance of needing an organ transplant [1]. However, regenerating soft tissues is challenging because of their multicellular population and complicated stratified structural properties [2,3]. Hydrogels possess significant potential in the realm of soft tissue engineering despite sharing structural and physicochemical features with the natural extracellular matrix (ECM) [4]. This involves the building of blood arteries, the heart, skin, nerves, muscles, and the liver. Hydrogels’ use in tissue engineering has grown exponentially since the development of bioprinting technology. Scaffolds of systematically complex structures can now be manufactured, and critical dimensional parameters, such as scaffold porosity and pore size, can be effectively controlled due to bioprinting’s ability to deposit different biomaterials (cells, growth factors, hydrogels, etc.) precisely and on demand in time and space. Consequently, the biomechanical development of tissue-engineered functional organs receives new hope with the combination of hydrogel-based biomaterials with 3D bioprinting technology. Many impressive advances have been made in the regeneration and restoration of soft tissues using 3D bioprinting hydrogel technology, which is now being used by an increasing number of academic researchers. Regenerative medicine is an emerging field; however, it has made remarkable progress in recent years. Its practices increase cell proliferation and differentiation and work to stimulate the body’s innate healing mechanisms. Multiple types of cells linked to tissue regeneration are delivered to the damaged location, which aids in tissue repair that goes beyond the self-healing potential. To thrive and perform their functions, cells need to communicate with one another and with the ECM (the extracellular matrix). The cells successfully express their biological roles in the body; however, the body requires tissue engineering materials that mimic the extracellular matrix (ECM) during tissue regeneration. In the field of tissue engineering, among the most significant difficulties is the development of solutions that can restore native tissue-like forms and functions. To optimize the conditions for tissue repair, researchers have undertaken several efforts to learn more about the relationships between cells, scaffolds, and bioactive chemicals [5]. Hydrogels have received a lot of attention for their use in a wide variety of applications, especially in soft tissues, because of their adaptability in terms of synthesis and functionalization for regulated biodegradation and mechanical characteristics. Hydrogels’ three-dimensional network design may be easily adjusted to achieve the required physical properties by measuring and controlling such factors as crosslinking density, elastic modulus, and degradation rate [6,7]. Thus, the degradation time can be set to correspond with the pace at which the target tissue is being regenerated (Figure 1), and the form and flexibility can be matched to the target tissue. The biocompatibility of the material allows for additional bioactivity to be introduced via the combination of functions and the conjugation of several bioactive compounds. These features are desirable because they make it possible to build materials in line with the stated regeneration strategy for each tissue. More and more hydrogels are being made from organically generated polymers because of their biocompatibility and valuable biofunctions [7]. Recent years have seen the increasing use of cell culture systems as scaffolds in which cells are added, in conjunction with 3D materials, as a method of acquiring even more complex regeneration tissues with the addition of growth factors, biological signal factors (i.e., peptides), and medicines. Sodium alginate (SA) has emerged as a popular hydrogel and is now considered a promising material for use in soft tissue scaffolds [8,9]. Sodium alginate is a natural polysaccharide that originates from brown algae kelp or Sargassum. The molecules that make up the compound comprise mannuronic acid and guluronic acid, which are bonded together by (1→4) bonds. Sodium alginate also has the benefits of being inexpensive, non-cytotoxic, simple to work with, and fast to gel [10,11]. The produced hydrogels share numerous structural and physicochemical characteristics with the natural extracellular matrix [12], which renders them promising for enclosing cells in the highly hydrated three-dimensional environment required for the formation and repair of soft tissue architecture. However, SA has some disadvantages in the field of soft tissue engineering: (i) due to its scaffold’s lackluster mechanical qualities, it cannot offer adequate mechanical support in a demanding setting. (ii) it suffers from a deterioration that occurs gradually and cannot be stopped; (iii) it is unable to engage with cells and provide adequate adhesion sites for cells [13,14]. Since sodium alginate hydrogels are used for tissue engineering, the absence of these flaws is crucial. Many unique physical, biological, and chemical characteristics of the substance (including its mechanical stiffness, swelling, disintegration, cell adherence, and its combination with bioactive compounds to provide a delayed release of growth factors) are the targets of physical and chemical modification techniques used by researchers [15,16]. This allows sodium alginate to better meet the diverse functional requirements of tissue engineering.
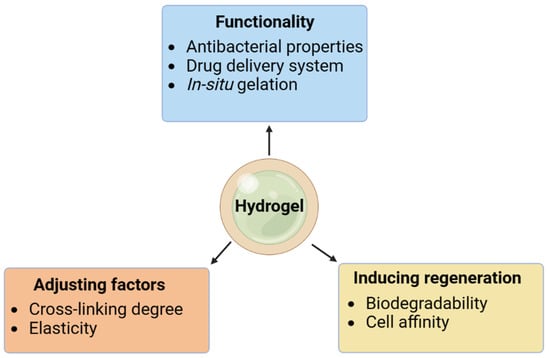
Figure 1. Parameters for optimal hydrogels.
2. Characterization of Sodium-Alginate-Based Hydrogels
Alginates are derived from brown seaweeds (Phaeophyceae), in which the polysaccharides play a crucial role in the thalli’s structural integrity. The alginate polysaccharide is linear in shape and composed of alternating blocks of homopolymeric (MM or GG) and heteropolymeric (MG) 1,4-linked -d-mannuronic acid (M) and -l-guluronic acid (G) units. The amount of M- and G-units and the block structure of seaweed are determined by its species, geographic location, season, vegetative phase, and the collected fraction of the algae species. Mannuronan C-5 epimerase [118] is responsible for controlling the M/G ratio in algae. Alginate hydrocolloids’ gelling qualities are based on the distribution of M- and G-units, as well as the counter ions present, whereas the hydrocolloids’ viscosity is dictated by their average molecular weight. Hard, inflexible gels are typical of those with a low M/G-ratio, whereas soft, malleable gels are typical of those with a high M/G-ratio. Brown seaweed and bacteria are both viable sources for the hydrogel polymer sodium alginate (SA). It is used in tissue engineering and for the targeted distribution of proteins and medicines [119,120] due to its biodegradability and high biocompatibility. Alginate is now known to be a type of linear copolymer in which M and G blocks are connected by 1,4-d-mannuronate and 1,4-l-guluronate residues, respectively. Gels made from l-guluronate in alginic acid are strong but brittle, while gels formed from d-mannuronate in alginic acid are weaker but more flexible [121]. The heteropolymer structure of alginic acid is linear. Because of their high G-content, low M: G alginates have found widespread use in a variety of fields, including environmental remediation, biomedicine, pharmaceuticals, food additives, and industry [122]. Medical applications such as drug administration and regenerative therapy benefit greatly from hydrogels’ capacity to (over time) breakdown into physiological metabolites under specific conditions. Hydrogels that mimic natural extracellular matrices and cell adhesion surfaces are useful for such applications because they facilitate the deployment of cells and their subsequent proliferation [123]. MG heteropolymeric blocks are interspersed with M and G homopolymeric blocks in naturally occurring alginates, which are linear polysaccharide chains. The food industry was not the only one to benefit from industrial alginate manufacturing; the industrial and medicinal sectors benefited as well [124]. The pharmaceutical industry uses this for a wide variety of purposes, including cancer treatment, protein, and cell delivery, and oral or controlled-release delivery [125]. Water content is intimately related to various hydrogel characteristics. The hydrogel is mostly water, and this water can be further broken down into two groups: waters that are highly associated and waters that are weakly related [126]. This categorization is based on the strength of the hydrogen bonds between water molecules and the alginate matrix. Some water molecules contact the hydrophilic groups in alginate for long periods, while other waters reside in the macropores, where they can move freely and only weakly interact with the polymers. In addition to providing information on the presence and size of the macropores, the latter macropore waters interact with the enclosed payload. Therefore, finding and researching these seas is crucial. Water molecules in hydrogels that are restricted and involved in strong hydrogen bonding to the polymer are resistant to freezing below 0°, making this a useful criterion for classifying (and detecting) different types of hydrogel waters. Other (biological) settings also exhibit this tendency [127,128]. The water in hydrogels can be divided into three categories: (1) water that does not freeze (highly bonded alginate), (2) water that has a freezing point like bulk water, and (3) water that freezes at a lower temperature inside the hydrogel [129,130,131]. Alternatively, hydrogel waters can be classified based on their mobility: water that is immobile, owing to strong alginate binding, is called “bound” water; water that is dynamic due to the absence of binding is called “free” water; and water that exhibits intermediate mobility is called “transient” water. Water is said to be “free” when it can move through or around the matrix (tissue) with minimal resistance and without interacting much with the alginate [132,133]. Bound water is water that is firmly attached to the matrix (in this case, alginate) and cannot move or freeze. Entrapped water is water that is encased by the structural features of the matrix but has weaker or temporary interactions with the matrix, giving it intermediate mobility. The mobility of these water molecules is restricted (in comparison to “free” water) [134]. Depending on the proximity and diffusion rates of the water pools, there may be an interchange between different pools. One advantageous element of this is that the relative amounts of these various hydrogel fluids reflect structurally and functionally significant characteristics such as the hydrogel mesh size and macropore size, with macropores filled with encapsulated but not tightly linked water molecules.
2.1. NMR Spectroscopy
NMR spectroscopy is well known for its application in liquid or solution states. Rapid thermal isotropic motions experienced by tiny soluble molecules in the solution state average out all orientation-dependent nuclear magnetic interactions. The resulting NMR spectra from a solution have a high signal-to-noise ratio because only isotropic components interact with it. Molecules in a “solid” state cause issues because they cannot tumble rapidly due to their size and restricted motions. Since “solid-state” NMR experiments do not involve small, dissolved molecules, the presence of orientation-dependent nuclear and internuclear interactions (such as anisotropic and dipolar interactions) is revealed. However, the resolution loss, decreased sensitivity, and difficulty in detecting individual atomic sites due to line broadening are all costs associated with these interactions, which provide insight into the local geometric and electronic structure [76]. The NMR spectra of most materials are broad and weak without line-narrowing procedures, which severely restrict the amount of information that can be gleaned from this method. Several methods have been created, however, to recover sharpness and sensitivity. Magic angle spinning (MAS) is frequently used in conjunction with solid-state NMR to dampen the dominant anisotropic interactions in the solid state. As part of this method, the sample is rapidly rotated at an angle of 54.74 degrees relative to the NMR instrument’s static magnetic field. If the MAS frequency is higher than the amplitude of the interaction, then the unwanted line-broadening interaction will be completely suppressed. As a result, the isotropic chemical shift frequencies observed in liquid-state NMR spectroscopy are shown to occur at the same frequencies in the solid-state NMR spectrum. The increasing speed of MAS has vastly improved the capabilities of current solid-state NMR. Since solid-state NMR spectroscopy using MAS-based techniques can provide in-depth molecular information without causing any damage or harm, it has become widely used in the pharmaceutical and biomedical industries [135,136,137,138,139]. MAS NMR provides structural and molecular dynamical information in a variety of non-crystalline environments, including amorphous and gel-like ones, in which other typical solid-state techniques fall short. Table 2 summarizes some important distinctions between solid- and liquid-state NMR.
Table 2. The table below is a summary of the main distinctions between solid- and solution-state NMR [140].
| Solid-State NMR | Solution-State NMR | |
|---|---|---|
| Type of sample | All physical states are possible | Only hydrolyzed gels |
| Sample preparation | Preparation (levels of hydration) is straightforward and manageable | Acid hydrolysis makes the preparation process lengthy |
| Restoration of samples | Yes | No |
| Limitations concerning hydrogels | Low sensitivity and resolution | Resolution depends on the solubility |
| Obtained information | Structure and dynamics of intact hydrogel | Chemical structure and composition |
2.2. Advantages of NMR Spectroscopy
The development of nuclear magnetic resonance (NMR) spectroscopy has been one of the most important contributions to the field of analytical science in recent decades. NMR has been used to study everything from a single cell to entire organs and tissues in both the biological and nonbiological sectors. Strong and consistent magnetic fields are needed for NMR. The magnitude of a magnet’s pull is expressed in tesla or megahertz. For NMR to work, the magnetic field strength must be represented by a reference nucleus. However, there is a risk of overexposure to radiation associated with the widespread use of electromagnetic spectra in healthcare and dentistry for the detection of abnormalities, fractures, and the monitoring of healing tissues. However, prolonged exposure to X-ray radiation can have negative consequences, such as cellular damage, even though it is painless and quick. In recent years, a plethora of cutting-edge analytical technologies that can provide pinpoint results with minimal tissue injury has emerged. In the 1940s, scientists made the initial discovery of nuclear magnetic resonance (NMR) [141].
2.3. Surface-Enhanced Raman Spectroscopy
Alginic acid is the major structural polysaccharide present in all brown seaweeds (Phaeophyta); it is a linear copolymer of β-D-mannopyranuronic acid (M) and α-L-gulopyranuronic acid (L) linked 1→4, which are arranged in homopolymeric and heteropolymeric blocks. There was no link found between M/G ratios and block composition in alginates, and the proportion of uronic acids in each species or tissue type varied widely. The characterization of alginic acid samples and block fractions using vibrational spectroscopy revealed that the FT-IR spectra of the homopolymannuronic and homopolyguluronic acid fractions displayed distinctive bands [142]. Raman spectroscopy has been used to identify alginates, as described by Pereira et al. [143], and IR, Raman, and NIR spectroscopies and chemometrics have been used to determine the M/G ratio in alginic acid, as reported by Salomonsen et al. [144]. Alginic acid salts have been reported as model compounds for use in the Raman spectroscopy study of biofilm matrix [145]. However, the high fluorescence of biological systems can mask the vibrational signals, and the low concentration of the samples results in poor spectra, limiting the utility of Raman and IR spectroscopies. Vibrational spectroscopy, which is enhanced by metal surfaces, has the potential to address these limitations since it may be used with low concentrations of analytes and because the effect of metal nanoparticles suppresses the inherent fluorescence of the materials [146]. In addition, measurements can be performed in aqueous conditions when metal colloids are used, which may facilitate conformational investigations of these macromolecules. The analytical method of surface-enhanced Raman spectroscopy (SERS) is particularly useful for elucidating the molecular structures of complicated substances [147]. Raman signals of molecules can be amplified by the electromagnetic field surrounding all the nanoparticles, which may increase the total vibrational signal by as much as 106 times. The first stage in Raman amplification was accomplished by Fleischmann et al. [147] using an electrochemical method with surface-adsorbed molecules of pyridine in a silver electrode. The detection of proteins, amino acids, peptides, and other biomolecules at concentrations as low as 1012 to 1014 M using SERS has now been reported in many publications [148]. Schmid et al. [149] recently examined alginate samples containing Ag colloids by tip-enhanced Raman spectroscopy.
2.4. Methodology of Sodium Alginate Hydrogel
Hydrogels made from alginate are just one example of biomaterial engineering that has benefited from the ever-expanding field of material science. The internal and diffusive gelling that occurs during construction is vital to many aspects of the final product. Gelation proceeds in two sequential reagents, with calcium ions becoming increasingly prominent within the body of the alginate, after a calculated injection of the calcium chloride solution into the alginate barrage. Numerous critical factors depend on the internal and diffusive gelling that occurs during building. Scaffolding makes it hard to maintain control [150]. The second approach involves using a double-nozzle procedure attached via a triad method of stopcock to manipulate the calcium chloride solutions and the alginate until the desired pliable hydrogel is achieved [151]. The technique’s main strength is how simple it is to generate a gel-like state consistently. Having a mechanical duty is just one of the many reasons why institutions can be utilized to make sodium alginate hydrogel scaffolds. To facilitate the process of alginate hydrogel injection, it is common to practice combining the alginate and calcium ions mechanically, as in the case of using a homogenization technique to combine the calcium gluconate solution and the marine alginate. Another technique involves subjecting the alginate solution to a barrage of divalent ions (a calcium chloride solution, for instance), which then facilitates the diffusion of calcium ions into the material’s core during the gelation process, beginning on the alginate’s outer surface. Using this method, one can create tissue scaffolds with a complicated architecture by manipulating alginate in a variety of fabrication procedures. However, bio-fabrication processes rely heavily on the presence of biomolecules and living cells, and the concentration of calcium ions is seen as an important goal for both [151,152]. Customized hydrogel materials also have various applications outside chemical catalysis, including nano-engineering, nanomedicines, nano-energy, and the visualization of reactions in aqueous media.
This entry is adapted from the peer-reviewed paper 10.3390/gels9050430
This entry is offline, you can click here to edit this entry!
