Storage of hydrogen in a liquefied form makes it possible to achieve a greater density than in the case of compressed storage. In a liquefied form, H2 is stored at atmospheric pressure and, therefore, it is possible to have a greater unit volume than in the case of compressed hydrogen. A similar storage method is already used at spaceports and is well-practiced.
An alternative way to store hydrogen is its chemical bonding or transformation into another substance. So, recently, methods of transporting and storing hydrogen in the form of a liquid organic hydride, or in the form of ammonia, have been developed [
60,
61]. The storage of these media is devoid of the disadvantages of storing hydrogen in compressed and liquefied form; however, the main disadvantage of this storage method is the need to convert hydrogen, which significantly increases costs. Thus, storage in the form of ammonia requires the construction of terminals for the production of ammonia, including an air separation plant.
An alternative and promising way of storing hydrogen is its pumping into natural underground cavities (e.g., salt caverns). This technology is widely used to store natural gas and is characterized by minimal investment, though geographical limitations minimize the usability of this storage method.
3. Hydrogen Transportation Methods
The main challenge to the widespread use of hydrogen transportation methods is a significant difference in hydrogen properties when compared with hydrocarbons (primarily boiling point and molecular weight).
Pipelines are one of the promising ways to transport hydrogen to consumers; however, the use of conventional gas pipelines intended for natural gas is not advisable because of the below reasons:
-
high potential for embrittlement of hydrogen pipeline steel and welded joints;
-
leaks of transported hydrogen through pipeline walls because of diffusion;
-
high hydrogen compression costs.
Potential solutions include using fiber reinforced polymer (FRP) pipelines for hydrogen distribution [
69].
The use of existing gas pipelines for pumping hydrogen is possible, but only in the case where hydrogen is mixed with natural gas (not more than 20%). This will enable the use of environmentally friendly fuel to reduce natural gas consumption, without the need to create new infrastructure. The restriction regarding fuel fractions is caused by the need to change the design of equipment used by main gas consumers (primarily gas turbine power units) due to the growing combustion temperature and burning velocity [
70].
The United States has an extensive network of more than 1600 miles of dedicated hydrogen pipeline [
71]. Hydrogen produced through clean pathways can be injected into natural gas pipelines, and the resulting blends can be used to generate heat and power with lower emissions than using natural gas alone.
Europe is taking the lead globally with pipelines planned on and offshore. The recently announced H
2Med Barcelona–Marseille subsea hydrogen pipeline is budgeted to cost around USD 2.1 billion for a stretch of 450 km, and it was recently announced that it will be extended to Germany too [
72].
Another way to transport compressed hydrogen is ground and sea transport. To date, it involves using vehicles with a maximum capacity of up to 1 ton of hydrogen [
73].
A low density of hydrogen is the cause of high costs of compressed hydrogen transportation. This problem can be solved by hydrogen transportation in a liquefied form. Due to the higher density of liquified hydrogen, it becomes possible to transport more fuel. An obvious disadvantage of this method is the need to provide low temperatures during transportation, which requires significant energy costs.
Transportation of liquid hydrogen is carried out by tank trucks with a capacity of 25 m3 and 45 m3. Hydrogen liquefaction is a highly energy-consuming process and, therefore, it is expensive, but transportation costs for liquid hydrogen are minimal and are roughly the same as the cost of delivery through pipelines.
A distinctive feature is that hydrogen is liquefied at a temperature of −253 °C, and special cryogenic tanks are necessary for its storage to minimize hydrogen losses. To this end, there are studies of materials, and aluminum tanks and containers of synthetic materials can be used as advanced technology.
In 2021, Kawasaki Heavy Industries obtained approval in principle from Nippon Kaiji Kyokai for a large, 160,000 m
3 liquefied hydrogen carrier [
74]. The carrier is designed to transport cryogenic liquefied hydrogen, cooled down to a temperature of –253 °C and reduced to one eight-hundredth its initial volume, by sea in large amounts on each voyage, helping to reduce hydrogen supply costs.
Railway transport for transportation of liquid hydrogen has rather a restricted application because of the limited railway network. In cryogenic railway tanks, hydrogen loss is about the same as in the case of tank trucks. With single chilldowns in tank trucks, up to 15% of hydrogen is lost, while losses associated with bad thermal insulation are 0.5% per day of the transported hydrogen amount.
There are also options to transport hydrogen using carriers, which can be hydrogen chemical compounds, such as ammonia or hydrocarbons. They go into chemical reactions to produce hydrogen. For example, an alternative option for transporting hydrogen is its conversion to ammonia or another form, such as a liquid organic hydrogen carrier (LOHC), to transport hydrogen in usual ways without significant costs to maintain the physical form and with minimal leaks. At a normal temperature, ammonia is liquefied at a pressure of 1.0 MPa, and it can be transported by pipes and stored in a liquid form (ammonia’s liquefaction temperature is −33 °C). Hydrogen is produced from ammonia through catalytic decomposition, with 5.65 kg of ammonia needed to produce 1 kg of hydrogen. However, a key drawback of this transportation method is the need to create ammonia/liquid organic hydrogen carrier plants with further decomposition at consumers.
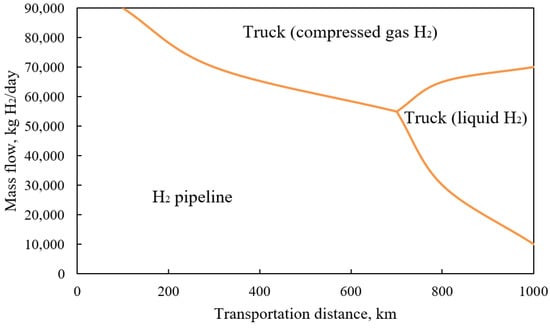
The choice of a hydrogen delivery option is primarily based on transportation costs. They depend on many factors from the amount of transported fuel to the distance between the manufacturer and the consumer. They affect both the capital cost of means of transportation (i.e., pipelines or ground transport) and operating costs (i.e., electricity costs for pumping hydrogen or truck fuel). Figure 1 presents tips on how to choose the most cost-efficient methods of hydrogen transportation over small and long distances, depending on the transported amount.
Figure 1. Recommendations for choosing an economically viable method of transporting hydrogen, depending on the distance and consumption.