Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Electroencephalography (EEG) signals are the primary source for discriminating the preictal from the interictal stage, enabling early warnings before the seizure onset. Epileptic seizure prediction systems face significant challenges due to data scarcity, diversity, and privacy.
- epilepsy
- seizure prediction
- preictal
1. Introduction
Epilepsy is a chronic neurological dysfunction syndrome characterized by repeated seizures induced due to irregular and excessive brain activity. Characteristics of seizure involve loss of consciousness, disruption of movement, and other cognitive malfunctions [1]. Epilepsy poses a severe disease burden; 70 million people are affected by epilepsy worldwide, according to the World Health Organization (WHO) survey, and about 20 million new epileptic patients are recorded each year [2]. Up to 70% of epileptic patients’ conditions are medically manageable using Anti-Epileptic Drugs (AED), whereas the conditions of 30% of people with epilepsy are unmanageable due to the unpredictability of their seizures [3]. Thus, epileptic seizure prediction has become extremely important to be able to save patients from seizures before they occur. Electroencephalograms (EEGs) record the brain’s electrical activity, serving as an analytical and diagnostic tool for epilepsy. EEGs are a widely used signal for measuring electrical, metabolic, or clinical changes in brain activity in order to observe the transition from the non-seizure state to the seizure state. EEG recordings of epileptic patients are categorized into multiple consecutive stages depending on the occurrence of seizures [4]. The preictal stage refers to that stage occurring before the onset of a seizure; the ictal stage refers to the phase while a seizure is occurring; the postictal stage refers to the period after a seizure; and finally, the interictal stage denotes the seizure-free period between the occurrence of two seizures [5]. Seizure onset [6] implies the actual generation of clinical seizures in the cortex. Figure 1 illustrates the epilepsy stages.
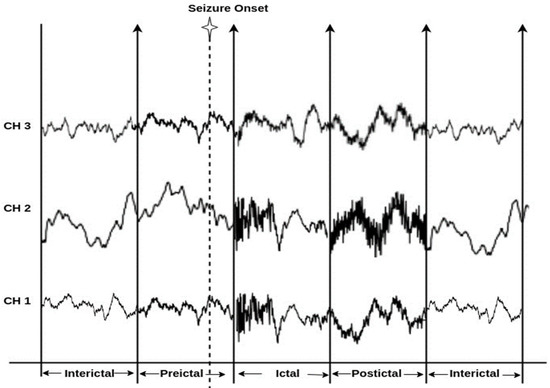
Figure 1. Diagram of epilepsy stages.
Epilepsy is a life-threatening disorder due to the occurrence of unexpected seizure episodes, creating a high psychological and social impact on epileptic patients. To promote quality of life, the prediction of epileptic seizures is necessary in order to control impending seizures [7]. It is essential to devise a potential seizure prediction system for patients who cannot be treated using medication. When processing epileptic EEG signals, seizure detection and prediction are modeled as classification tasks. Small seizure durations in EEG recordings indicate that the interictal state is comparatively longer than the ictal state. Hence, the seizure detection task attempts to differentiate the ictal state from the interictal state. In the subsequent identification of the preictal interval, seizure onset prediction critically supports the early medical diagnosis of epileptic seizures in patients.
Despite the abundance of epilepsy research activities, researchers have envisioned the possibility of performing accurate seizure prediction and interceding before the onset of seizure indications [8]. Conventional works utilize handcrafted features marked on EEG signals to locate the preictal state before seizure onset. However, manual feature extraction leads to inaccurate seizure prediction due to information loss and extends the warning time. Epileptic seizure prediction research works have employed various models, such as machine learning and signal processing [9]. Machine-learning-based seizure prediction and medical diagnosis have been accompanied by privacy concerns due to the considerable sensitivity of health data. In addition to the patient characteristics included in the health data, the processing of diagnosis results is also sensitive; hence, privacy becomes a major constraint. Even though research into machine-learning-based diagnosis has investigated different encryption methods, medical diagnosis systems are confronted with the challenge of low efficiency while needing to achieve high levels of privacy. The concept of Federated Learning (FL) [10] has emerged as a potential solution, and is able to overcome privacy issues while training machine learning models using the data of edge devices distributed worldwide.
Developing a high-performance model is crucial in order to provide a reliable, real-time medical diagnosis. The FL model protects users’ data by transferring only model parameters trained locally on the client device, instead of transferring clients’ data to the cloud. Owing to the shortage of clinical experts and the high cost of manual diagnosis, adopting FL improves the quality of healthcare service without an expensive diagnosis. In the FL, local model learning and decision making are performed by leveraging local data and global model knowledge, whereas the global shared model receives updates from distributed local models trained on various data. Hence, the main aim of the early epileptic seizure prediction system is fulfilled by the FL model while ensuring privacy, minimal latency, and minimal power consumption.
2. Deep-Learning-Based Epileptic Seizure Prediction Approaches
A patient-specific seizure prediction approach has been reported [11] adopting the CNN model to categorize the preictal stage on the basis of EEG and iEEG signals. Short-time Fourier transform is used to perform raw EEG data preprocessing with minimal effort in the feature engineering process. A seizure prediction framework [12] using Long Short-Term Memory Networks (LSTM) has been developed to analyze the preictal state on the basis of EEG signals. This LSTM-based seizure prediction system utilizes a broad range of feature extraction methods, namely, in the frequency and time domains, graph using theoretical measures and EEG correlation to impart solid ictal prediction performance. Another seizure prediction methodology has been presented [13] that was designed to distinguish between the preictal and interictal phases using CNN, and data equalization was performed in order to overcome the trial imbalance problem. This model utilized common spatial patterns and wavelet packet-based decomposition feature extractors to extract the temporal–frequency characteristics of EEG signals. Another epileptic seizure occurrence prediction model was presented [14] in which the multi-view CNN model was exploited to attain different views of EEG signals. This model acquires discriminative and adequate feature representations from EGG data using time- and frequency-domain methods. A seizure prediction approach [15] was developed in which LSTM was utilized to differentiate the preictal state from the interictal and ictal states on the basis of EEG signals. In this approach, a stacked Bi-LSTM network is built to achieve better seizure prediction performance before seizure onset.
An end-to-end patient-specific model has been reported [16] in which CNN is employed to predict seizures before seizures. In this method, the CNN network is implemented using one-dimensional (1D) and two-dimensional (2D) kernels in the initial and final stages of the convolution and max-pooling layers to attain greater accuracy. The efficient seizure prediction approach [17] relies on CNN to extract features automatically and to classify the preictal and interictal segments of the EGG. In this approach, EEG channel optimization is conducted using the channel reduction technique in order to predict seizures on the basis of EEG signals. A patient-specific seizure prediction system has been reported [18] in which electrocardiogram (ECG) features, particularly the time and frequency features from the RR series, are examined by means of recurrence quantification analysis. Furthermore, it exploits the Support Vector Machine (SVM) model to classify preictal and interictal segments. To identify the preictal state from the EEG signals, the research reported in [19] investigated the HRV features of ECG signals while considering the frequency- and time-domain features for the recognition of each seizure. Early changes in the EEG and HRV features assist in characterizing the preictal and interictal states in drug-resistant epilepsy patients. The research reported in [20] developed an ANFIS-based seizure prediction system for patients affected by Parkinson’s disease. By modeling the ANFIS for the purpose of EEG signal analysis, the starting point of seizure onset could be detected, thus supporting real-time seizure prediction. However, performing real-time medical diagnosis on the basis of the examination of a single modality of EEG input data alone is ineffective due to the lack of exploration of different inputs.
3. Hybrid-Learning-Based Epileptic Seizure Prediction Approaches
A generalized deep learning framework has been reported [21] for seizure prediction employing the CNN-LSTM architecture. Initially, in this framework, Short-Time Fourier Transform (STFT) is applied to effectively carry out EEG signal preprocessing. Then, the features of sequential EEG segments are captured using spectral, spatial, and temporal methods, and the preictal EEG segments are distinguished from the interictal EEG segments, with high prediction performance. An effective patient-specific seizure forecasting method has been described [22] in which the Deep Convolutional Neural Network (DCNN) and Bidirectional LSTM (Bi-LSTM) models are employed to analyze the temporal and spatial features of raw EEG signals. Subsequently, this method enables a Deep Convolutional Auto-Encoder (DCAE)-model-based supervised learning method with transfer learning and channel selection to diminish the training time and computation load while predicting the seizure events. In the research work reported in [23], the EMD and DWT methods were employed to convert the raw EEG signals into the extracted features, which were then provided as the input to the classification models, specifically the Decision Tree, and their approach was evaluated using the Bonn EEG dataset. The epileptic seizure prediction system reported in [24] consisted of a method in which LSTM and CNN were combined, and a Long-term Recurrent Convolutional Network (LRCN) model was presented. The LRCN design was used to identify preictal segments by analyzing the spatial and temporal information in an EEG sequence belonging to the CHB-MIT dataset. A novel epileptic seizure prediction approach has been presented [25] in which a hybrid DenseNet-LSTM model is employed for forecasting patient-specific epileptic seizures. The hybrid DenseNet-LSTM model integrates the DCNN and LSTM networks. Furthermore, it applies Discrete Wavelet Transform (DWT) to the EEG signals, transforms them using CNN, and then classifies preictal and interictal states using LSTM.
An epileptic seizure forecasting method has been developed [26] that is able to predict the preictal stage of seizure activity. This method encompasses a series of processes, including Empirical Mode Decomposition (EMD), for preprocessing EEG signals, Generative Adversarial Network (GAN), to overcome class imbalance issues, and CNN, to perform automated optimal feature extraction, while LSTM is exploited to robustly distinguish preictal and interictal segments. An epileptic EEG recognition approach [27] utilizes the improved residual network architecture to diagnose epileptic EEG, and different states of epileptic EEGs are automatically labeled. This improved residual network is an independent Recurrent Convolutional Neural Network (RCNN) composed of a One-Dimensional CNN to preprocess the essential features of EEG and an Independent Recurrent Neural Network (indRNN) to learn the correlations among EEG signal sequences and differentiate different ictal periods. Deep ensemble learning has been proposed [28] for epileptic seizure forecasting, where EMD is incorporated to remove noise and GAN to generate synthetic preictal stages. Subsequently, it exploits three-layered customized CNN to extract a comprehensive feature set and SVM, CNN, and LSTM in order to enable ensemble classifiers using Model-Agnostic Meta-Learning (MAML) to differentiate between preictal and interictal states. A new neuromorphic computing approach has been reported [29] in which the Gaussian random discrete encoder is employed to create spike sequences for the input EEG data. The combination of the energy-efficient SNN and CNN is able to perform seizure prediction by leveraging the potential advantages of each model. The seizure prediction approach [30] mitigates the need for higher computation consumption in information fusion by adopting a Graph CNN (GCN) that explores the graph structure of EEG signals. Designing a simple network architecture with node and edge features predicts seizures on the basis of scalp EEG signals. Despite this, the generalized graph structure can result in the medical misdiagnosis of individual patients, because the edge features in the graph are sensitive to differences among patients.
It can be concluded from the above literature analysis that there are different models for epileptic seizure prediction, and new solutions are emerging. However, there are several research directions in patient-specific preictal state detection leveraging early diagnosis that are not pioneering; therefore, several constraints must be resolved in order to achieve reliable seizure prediction in real time. For decision making in environments characterized by data scarcity, extracting other patients’ preictal information has not received adequate attention. Additionally, automated personalization on the basis of small EEG patterns without handcrafted features remains an emerging field of research. The fusion of multiple seizure-indicating features, such as EEG signals, ECG signals, and clinical records, requires further research for accurate seizure prediction. In deep learning, the handling of data scarcity and the preservation of privacy in small sensitive medical datasets have not been well studied.
This entry is adapted from the peer-reviewed paper 10.3390/s23146578
References
- Beghi, E. The epidemiology of epilepsy. Neuroepidemiology 2020, 54, 185–191.
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701.
- Kuhlmann, L.; Lehnertz, K.; Richardson, M.P.; Schelter, B.; Zaveri, H.P. Seizure prediction—Ready for a new era. Nat. Rev. Neurol. 2018, 14, 618–630.
- Mula, M.; Monaco, F. Ictal and peri-ictal psychopathology. Behav. Neurol. 2011, 24, 21–25.
- Wang, Y.; Li, Z.; Feng, L.; Zheng, C.; Zhang, W. Automatic detection of epilepsy and seizure using multiclass sparse extreme learning machine classification. Comput. Math. Methods Med. 2017, 2017, 6849360.
- Jehi, L. The epileptogenic zone: Concept and definition. Epilepsy Curr. 2018, 18, 12–16.
- Usman, S.M.; Khalid, S.; Akhtar, R.; Bortolotto, Z.; Bashir, Z.; Qiu, H. Using scalp EEG and intracranial EEG signals for predicting epileptic seizures: Review of available methodologies. Seizure 2019, 71, 258–269.
- Assi, E.B.; Nguyen, D.K.; Rihana, S.; Sawan, M. Towards accurate prediction of epileptic seizures: A review. Biomed. Signal Process. Control 2017, 34, 144–157.
- Patel, V.; Tailor, J.; Ganatra, A. Essentials of Predicting Epileptic Seizures Based on EEG Using Machine Learning: A Review. Open Biomed. Eng. J. 2021, 15, 90–104.
- Aledhari, M.; Razzak, R.; Parizi, R.M.; Saeed, F. Federated learning: A survey on enabling technologies, protocols, and applications. IEEE Access 2020, 8, 140699–140725.
- Truong, N.D.; Nguyen, A.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.; Ippolito, S.; Kavehei, O. Convolutional neural networks for seizure prediction using intracranial and scalp electroencephalogram. Neural Netw. 2018, 105, 104–111.
- Tsiouris, Κ.Μ.; Pezoulas, V.C.; Zervakis, M.; Konitsiotis, S.; Koutsouris, D.D.; Fotiadis, D.I. A long short-term memory deep learning network for the prediction of epileptic seizures using EEG signals. Comput. Biol. Med. 2018, 99, 24–37.
- Zhang, Y.; Guo, Y.; Yang, P.; Chen, W.; Lo, B. Epilepsy seizure prediction on EEG using common spatial pattern and convolutional neural network. IEEE J. Biomed. Health Inform. 2019, 24, 465–474.
- Liu, C.L.; Xiao, B.; Hsaio, W.H.; Tseng, V.S. Epileptic seizure prediction with multi-view convolutional neural networks. IEEE Access 2019, 7, 170352–170361.
- Thara, D.K.; PremaSudha, B.G.; Xiong, F. Epileptic seizure detection and prediction using stacked bidirectional long short term memory. Pattern Recognit. Lett. 2019, 128, 529–535.
- Xu, Y.; Yang, J.; Zhao, S.; Wu, H.; Sawan, M. An end-to-end deep learning approach for epileptic seizure prediction. In Proceedings of the 2020 2nd IEEE International Conference on Artificial Intelligence Circuits and Systems (AICAS), Genova, Italy, 31 August–2 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 266–270.
- Jana, R.; Mukherjee, I. Deep learning based efficient epileptic seizure prediction with EEG channel optimization. Biomed. Signal Process. Control 2021, 68, 102767.
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339.
- Leal, A.; Pinto, M.; Henriques, J.; da GraçaRuano, M.; de Carvalho, P.; Teixeira, C. Preictal Time Assessment using Heart Rate Variability Features in Drug-resistant Epilepsy Patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA; pp. 6776–6779.
- Daher, A.; Yassin, S.; Alsamra, H.; Abou Ali, H. Adaptive Neuro-Fuzzy Inference System as New Real-Time Approach for Parkinson Seizures Prediction. In Proceedings of the 2021 4th International Conference on Bio-Engineering for Smart Technologies (BioSMART), Paris/Créteil, France, 8–10 December 2021; IEEE: Piscataway, NJ, USA; pp. 1–4.
- Shahbazi, M.; Aghajan, H. A generalizable model for seizure prediction based on deep learning using CNN-LSTM architecture. In Proceedings of the 2018 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Anaheim, CA, USA, 26–29 November 2018; IEEE: Piscataway, NJ, USA; pp. 469–473.
- Daoud, H.; Bayoumi, M.A. Efficient epileptic seizure prediction based on deep learning. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 804–813.
- Bekbalanova, M.; Zhunis, A.; Duisebekov, Z. Epileptic seizure prediction in EEG signals using EMD and DWT. In Proceedings of the 2019 15th International Conference on Electronics, Computer and Computation (ICECCO), Abuja, Nigeria, 10–12 December 2019; IEEE: Piscataway, NJ, USA; pp. 1–4.
- Wei, X.; Zhou, L.; Zhang, Z.; Chen, Z.; Zhou, Y. Early prediction of epileptic seizures using a long-term recurrent convolutional network. J. Neurosci. Methods 2019, 327, 108395.
- Ryu, S.; Joe, I. A Hybrid DenseNet-LSTM model for epileptic seizure prediction. Appl. Sci. 2021, 11, 7661.
- Usman, S.M.; Khalid, S.; Bashir, Z. Epileptic seizure prediction using scalp electroencephalogram signals. Biocybern. Biomed. Eng. 2021, 41, 211–220.
- Ma, M.; Cheng, Y.; Wei, X.; Chen, Z.; Zhou, Y. Research on epileptic EEG recognition based on improved residual networks of 1-D CNN and indRNN. BMC Med. Inform. Decis. Mak. 2021, 21, 100.
- Usman, S.M.; Khalid, S.; Bashir, S. A deep learning based ensemble learning method for epileptic seizure prediction. Comput. Biol. Med. 2021, 136, 104710.
- Tian, F.; Yang, J.; Zhao, S.; Sawan, M. A new neuromorphic computing approach for epileptic seizure prediction. In Proceedings of the 2021 IEEE International Symposium on Circuits and Systems (ISCAS), Daegu, Korea, 22–28 May 2021; IEEE: Piscataway, NJ, USA; pp. 1–5.
- Jia, M.; Liu, W.; Duan, J.; Chen, L.; Chen, C.P.; Wang, Q.; Zhou, Z. Efficient graph convolutional networks for seizure prediction using scalp EEG. Front. Neurosci. 2022, 16, 967116.
This entry is offline, you can click here to edit this entry!
