Aging is a normal process in the life of any species. Still, some individuals experience early or premature aging and, thus, advanced age-associated diseases impacting the quality of their life, accompanied by enormous economic and social burdens. Therefore, it would be rational to mitigate aging processes, not only to support healthy aging but also to hamper age-associated diseases. During aging, different functional systems are affected interactively. These include the central nervous system (CNS), cardiovascular system, immune system, and the gut ecosystem. Additionally, the musculoskeletal system is prone to progressive weakening, causing movement problems that could intensify the aforementioned complications and increase the risk of all types of dementia, including Alzheimer’s disease.
- aging
- neurodegeneration
- microglia
- neuroinflammation
- phytochemicals
- molecular target
- gut microbiota
- neural stem cell
1. Neuroinflammation and Microglia Dysregulated Genes in Alzheimer’s Disease and the Effects of Phytochemicals
Other key elements of the immune system in AD are complements C1q, C3, and C4 [24,25], along with TGFB2 [26] which regulates synapse pruning [27]. As shown in Figure 1, Aβ protein activates astroglia NFκb and releases the complement C3 which acts on neuronal complement C3a receptors (C3aR) and disrupts dendritic morphology and network functions in AD patients [28]. Other studies reported that complement C3 and C3aR1 are increased by aging, which results in inflammation and increases the permeability of the vascular structure (mediated by Ca++), impacting the blood-brain barrier (BBB) integrity associated with lymphocyte infiltration, and greater microglial activity [29]. Furthermore, as the expression of complement C3 and C3aR1 in human AD brain are correlated with cognitive decline, the inactivation of C3aR attenuates tau pathology, neuroinflammation, and neurodegeneration in mice models of AD [30].
Notably, aging is also associated with DNA hypomethylation and reactivation of transposon elements such as LINE1 and SINE which induce inflammation. Transposons are viral DNA that has been incorporated into the genome of different species and compromise almost 50% of the human genome. Epigenetic mechanisms, including DNA methylation, are responsible for suppressing their activity throughout the life of any animal. It has been shown that SIRT6 is one of the key genes in transposons inactivation via inducing DNA methylation, and its upregulation suppresses transposons reactivation leading to longer life in Drosophila melanogaster [37]. In humans, its overactive allele is associated with longer life, i.e., becoming a centenarian [38]. Fucoidan, a phytochemical of Atlantic brown algae, increases SIRT6 expression by nearly 14-fold [39], increases lifespan and is neuroprotective in D. melanogaster [40,41], and improves LPS-induced cognitive impairment in mice [42], thus a promising anti-aging phytochemical.
Besides SIRT6, SIRT1 expression is also downregulated by brain internal factors/toxins such as Aβ accelerating neuronal cell senescence, which can be attenuated by exogenous SIRT1 expression as well as aspirin (salicylic acid, a plant hormone) that upregulates SIRT1 expression [43]. Phytic acid was also shown to diminish the dysfunction of these players in cell culture experiments and the aged mice brain [44]. In addition, it has been shown that the active compounds of black chokeberry (Aronia melanocapa L.) decrease the expression of several inflammatory factors, including ITGB2/C3R, in neuronal cells [45]. As ITGB2 is upregulated in blood mononuclear cells of AD patients, 1α,25(OH)2-vitamin D3 (1,25D3) could promote Aβ phagocytosis by macrophages of AD patients and decrease inflammation in vitro [46].
Regarding TGFβ2, while its overexpression is reported in the neurons of patients with AD [26], in vitro studies uncovered that its expression is induced by toxic Aβs both in glial and neuronal cells. Interestingly, the increased TGFβ2 protein in the brain binds to the extracellular domain of Aβ precursor protein and activates a neuronal cell death pathway in AD, and the degree of TGFβ2-induced cell death is greater in cells that express a familial AD-related mutation in APP versus those that express the wild-type APP [26,47]. There is evidence that the expression of TGFβ2 can be downregulated by certain phytochemicals (e.g., withaferin A and active fractions of golden-flowered tea) in other diseases, suggesting their potential use in AD [48-50]. In neuronal tissue, curcumin exhibits anti-inflammatory effects and suppresses reactive gliosis in animal models of spinal cord injury through decreasing TGFβ1 and TGFβ2, along with proinflammatory cytokines such as TNF-α, IL-1β, and NF-κb [51]. High Mobility Group Box 1 (HMGB1), another gene linked to microglia function, is also involved in AD pathogenesis [52]. As HMGB1 is a known marker of neuroinflammation, and the serum level of HMGB1 is higher in AD [53], it may disrupt the BBB functions [54]. Tau oligomer also induces HMGB1 release promoting cellular senescence [55]. Quercetin and naringin are known to suppress HMGB1 and ROS or proinflammatory cytokines in other diseases [55,56]. Galangin could suppress the HMGB1/TLR4 bond mitigating astrocytic activation and neuroinflammation in rat brains [58]. In other studies, it was shown that HMGB1 expression is regulated by HDAC4&5 and miR-129 in brain cells [61,62]. As miR-129 is known to regulate neuronal migration in mice brains [63], choline (abundant in spinach) upregulates miR-129-5p expression in neural progenitor cells both in vitro and in vivo [64]. Other phytochemicals (e.g., gallic acid and sulforaphane) could influence HDAC4 or HDAC5, thus opening new windows for more studies in the treatment of AD and other neurodegenerative diseases. YAP and TAZ genes (members of the superfamily of ATP-binding cassette transporters) whose activities decline during aging are other key elements of cellular senescence or aging. As YAP/TAZ, through the suppression of cGAS/STING, preserve nuclear envelope integrity involving innate immunity and prevent aging, inhibition of STING (stimulator of interferon genes or TMEM173) prevents tissue aging and senescence-associated inflammation [65]. PQBP1-cGAS-STING pathway is also involved in Tau-induced microglia activation leading to brain inflammation [66]. It has been shown that supplements containing NAD+ (and its precursors abundant in mushroom, avocado, and cucumber) could reduce the expression of proinflammatory cytokines and mitigate microglia and astrocytes activation through decreasing cGAS-STING activity which finally attenuates neuroinflammation and cell senescence in a mouse model of AD [67]. The cGAS-STING signaling could be inhibited by Astin C (a cyclopeptide extracted from the plant of Aster tataricus) as well, which decreases the innate inflammatory responses triggered by cytosolic DNAs both in vitro and in mice [68]. Figure 1 and Table 1 show key affected genes and a summary of findings related to the effects of different phytochemicals with potential application in neurodegenerative diseases. However, there are other phytochemicals that exhibit neuroprotective effects via other mechanisms. For example, quercetin exhibits neuroprotective effects through the downregulation of α-synuclein protein aggregation in PD [14] and inhibits the fibril formation of Aβ proteins in AD [15]. Similarly, naringenin could alleviate the neurotoxic effects of Aβ in vitro associated with the downregulation of the expression of APP and BACE, reducing amyloidogenesis, and decreasing the phosphorylated tau level [16]. Several other lines of evidence also support the beneficial effects of naringenin in AD and PD [17], as well as the neuroprotective effects of Bacopa monnieri extract, with potential therapeutic applications in AD [18].
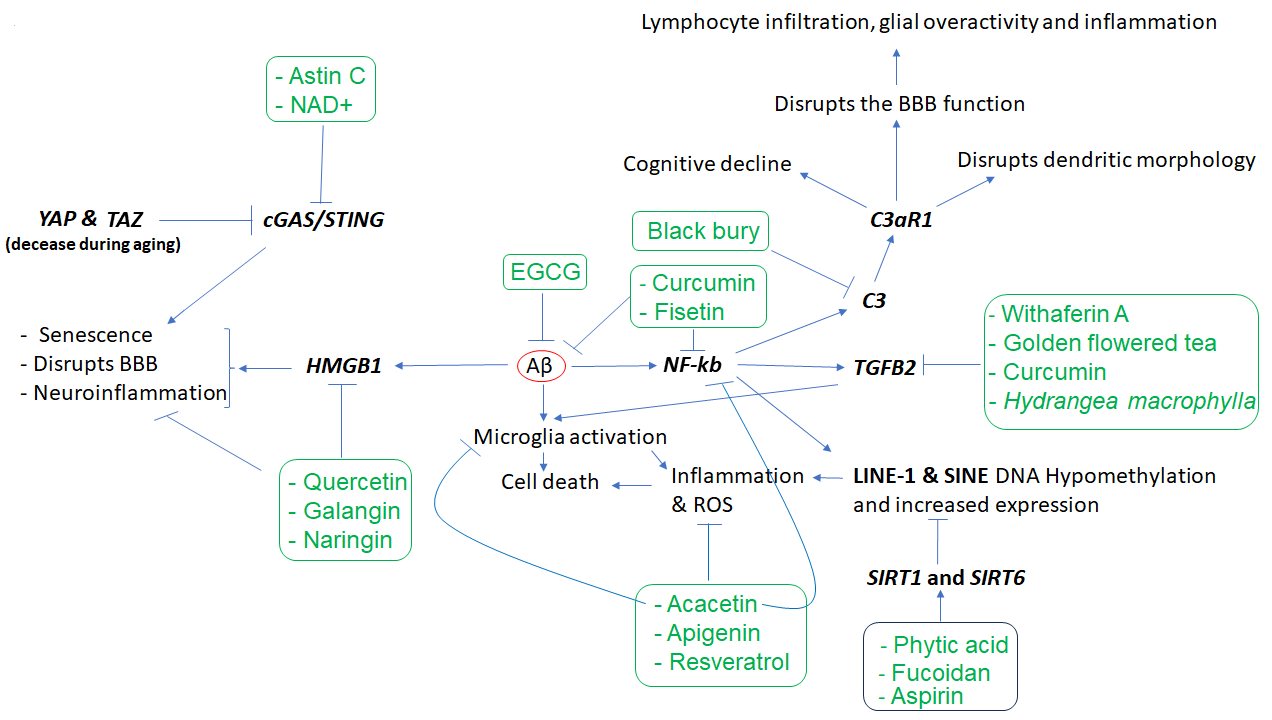
Figure 1. The cascade of events resulting from amyloid-beta (Aβ) accumulation in the brain and the phytochemicals that may mitigate the effects of these events. Aβ appears to play a central role in inducing inflammation, which is mediated by the overactivity of HMGB1, NKB, C3, C3AR, and TGFB2, as well as LINE-1 and SINE transposons, among others. However, several phytochemicals (inside rectangles) have the potential to target the affected genes, reduce inflammation, and mitigate the other cellular dysfunctions that contribute to neuronal death. In the figure, arrows indicate stimulation, while the T-shape marks denote inhibition. Genes are presented in bold.
| Phytochemicals or Nutrients | In Vitro and/or Animal Models | Effects | Mechanisms of Action | References |
|---|---|---|---|---|
| Anthocyanins (bilberry) | APP/PSEN1 transgenic mouse model of AD |
|
|
[19] |
| Cyanidin-3-O-glucoside (fruits and vegetables) | Mouse model of AD |
|
|
[20] |
| Fucoidan (Atlantic brown algae) |
D. melanogaster and mice |
|
|
[21][22][23] |
| Bioactive compounds in black chokeberry (Aronia melanocapa L.) | Neuronal cells and mice brain |
|
|
[24] |
| Curcumin | In vitro and in vivo |
|
|
[25] |
| Curcumin | Animal model of spinal cord injury |
|
|
[26] |
| Galangin | Rat brain |
|
|
[27] |
| Astin C (a cyclopeptide from the Aster tataricus plant) | Mice |
|
|
[28] |
| Apigenin (parsley and celery) | iPSC-derived neurons from patients with AD |
|
|
[29] |
| Resveratrol (grapes and berries) | Yeast and flies |
|
|
[30] |
| EGCG (tea polyphenol) | APP/PS1 mouse model of AD |
|
|
[31] |
| Acacetin (Robinia pseudoacacia plant) | MPTP-induced mouse model of PD and LPS-induced mouse model of neuroinflammation |
|
|
[32][33] |
| Phytic acid (plants and seeds) and aspirin | Neuronal cells and aged mice brain |
|
|
[34][35] |
2. Brain Neuronal Stem Cells (NSCs) in Aging and the Effects of Phytochemicals
In more recent studies, it has been shown that while key genes encoding basic transcription factors for stemness are active during embryogenesis, they are silenced in later life. However, concurrent increases in the expression of SOX2, OCT4, and KLF4 have been associated with recovering the lost epigenetic information in aged cells and the rejuvenation of several tissues, including neuronal cells in mice eyes [95]. Therefore, the induction of the expression of these genes was shown to be a promising therapeutic approach for the treatment of age-related diseases via recovering epigenetic memory [17,95]. Several phytochemicals are known to potentiate the expression of these genes. For example, di-(2-ethylhexyl) phthalate (DEHP), a C. vulgure phytochemical, increases SOX2 expression in hippocampal NSC of mice in vitro associated with increased cell growth rate [96]. Furthermore, sulforaphane (a phytochemical compound of broccoli), Withaferin A (a steroidal lactone from a medicinal plant), and Betulinic acid (a phytochemical from several tree bark extracts) could increase the expression of KLF4 in vitro [97-99]. Although many phytochemicals reduce the expression of OCT4, short-term low-dose ethanol treatment (1 week, 1-5 mM, equivalent to the blood concentration of ~0.0048-0.024%) increases OCT4 expression several folds in vitro [100]. Therefore, an appropriate combination of these phytochemicals with low-dose ethanol may help to increase the expression of these key transcription factors posited to be useful in tissue rejuvenation or regeneration.
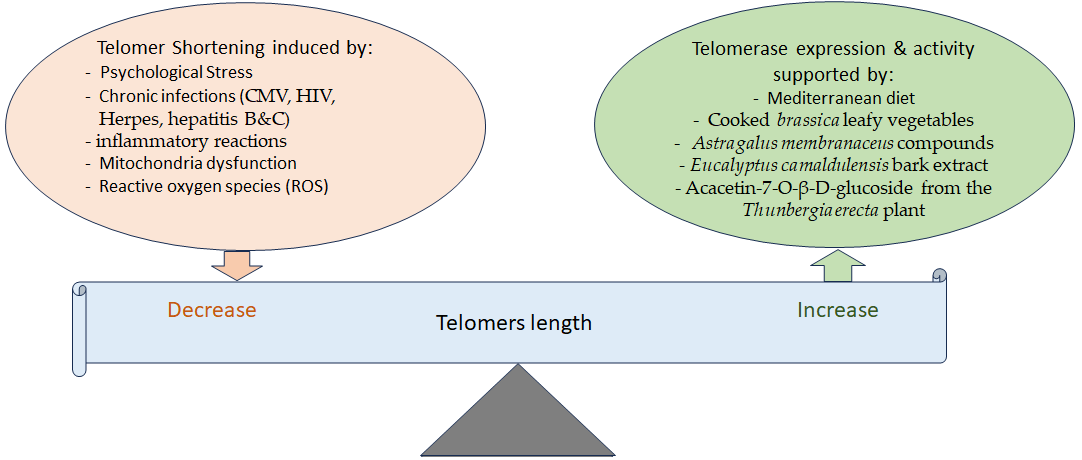
3. Telomere Attrition and Aging and the Effects of Phytochemicals
4. Gut Microbiome, the Dysfunction of Brain Microglia and Astrocytes in Brain Aging, and Phytochemical Effects
| Disease | Country | Increased | Decreased | Reference |
|---|---|---|---|---|
| PD | Finland | - | Prevotellaceae * | [61] |
| PD | USA | Blautia, Coprococcus, and Roseburia | [66] | |
| PD | Japan | Lactobacillus * | Clostridium coccoides * and Bacteroides fragilis * | [67] |
| PD | Russia | Lactobacillus *, Bifidobacterium, and Papillibacter cinnamivorans among others | Dorea, Bacteroides, Prevotella *, Coprococcus eutactus, and Ruminococcus callidus, among others | [68] |
| PD | China | Alistipes, Paraprevotella, Klebesiella, Sphingomonas, Acinetobacter, Aquabacterium, Desulfovibrio, Clostridium IV, Lachnospiracea incertae sedis, Butyricicoccus, Clostridium XVIII, and Nitrososphaera | Lactobacillus ¥ and Sediminibacterium | [69] |
| PD | Taiwan | Verrucomicrobia, Mucispirillum, Porphyromonas, Lactobacillus *, and Parabacteroides | Prevotella * (a genera of Prevotellaceae) * | [58] |
| PD | Italy | Lachnospiraceae | [70] | |
| AD | USA | Bacteroidetes *, Blautia®, and Alistipes© | Bifidobacterium ¥, Firmicutes, and Actinobacteria | [57] |
| AD | USA | Bacteroides *, Alistipes©, Odoribacter ¥, and Barnesiella | Lachnoclostridium, Butyrivibrio, and Eubacterium | [56] |
| AD | China | Bifidobacterium ¥, Sphingomonas, Lactobacillus, and Blautia® | Odoribacter ¥, Anaerobacterium, and Papillibacter | [63] |
| AD | Turkey | Bacteroides * and Prevotella | [64] |
5. Metabolic Disease, Caloric Restriction, Physical Exercise, and Aging
6. Chromosome X Inactivation and Neurodegeneration
7. Vascular System and Neurodegeneration
This entry is adapted from the peer-reviewed paper 10.3390/nu15153456
References
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472.
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129.
- Xu, H.; Jia, J. Single-Cell RNA Sequencing of Peripheral Blood Reveals Immune Cell Signatures in Alzheimer’s Disease. Front. Immunol. 2021, 12, 645666.
- Xiong, L.L.; Xue, L.L.; Du, R.L.; Niu, R.Z.; Chen, L.; Chen, J.; Hu, Q.; Tan, Y.X.; Shang, H.F.; Liu, J.; et al. Single-cell RNA sequencing reveals B cell-related molecular biomarkers for Alzheimer’s disease. Exp. Mol. Med. 2021, 53, 1888–1901.
- Zhang, L.; Silva, T.C.; Young, J.I.; Gomez, L.; Schmidt, M.A.; Hamilton-Nelson, K.L.; Kunkle, B.W.; Chen, X.; Martin, E.R.; Wang, L. Epigenome-wide meta-analysis of DNA methylation differences in prefrontal cortex implicates the immune processes in Alzheimer’s disease. Nat. Commun. 2020, 11, 6114.
- Pellegrini, C.; Pirazzini, C.; Sala, C.; Sambati, L.; Yusipov, I.; Kalyakulina, A.; Ravaioli, F.; Kwiatkowska, K.M.; Durso, D.F.; Ivanchenko, M.; et al. A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer’s Disease Points for Accelerated Epigenetic Aging in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 639428.
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Res. Rev. 2022, 81, 101743.
- Chiavellini, P.; Canatelli-Mallat, M.; Lehmann, M.; Gallardo, M.D.; Herenu, C.B.; Cordeiro, J.L.; Clement, J.; Goya, R.G. Aging and rejuvenation—A modular epigenome model. Aging 2021, 13, 4734–4746.
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e27.
- Smith, A.R.; Smith, R.G.; Condliffe, D.; Hannon, E.; Schalkwyk, L.; Mill, J.; Lunnon, K. Increased DNA methylation near TREM2 is consistently seen in the superior temporal gyrus in Alzheimer’s disease brain. Neurobiol. Aging 2016, 47, 35–40.
- Celarain, N.; Sanchez-Ruiz de Gordoa, J.; Zelaya, M.V.; Roldan, M.; Larumbe, R.; Pulido, L.; Echavarri, C.; Mendioroz, M. TREM2 upregulation correlates with 5-hydroxymethycytosine enrichment in Alzheimer’s disease hippocampus. Clin. Epigenet. 2016, 8, 37.
- Ozaki, Y.; Yoshino, Y.; Yamazaki, K.; Sao, T.; Mori, Y.; Ochi, S.; Yoshida, T.; Mori, T.; Iga, J.I.; Ueno, S.I. DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer’s disease. J. Psychiatr. Res. 2017, 92, 74–80.
- Wu, M.; Liao, M.; Huang, R.; Chen, C.; Tian, T.; Wang, H.; Li, J.; Li, J.; Sun, Y.; Wu, C.; et al. Hippocampal overexpression of TREM2 ameliorates high fat diet induced cognitive impairment and modulates phenotypic polarization of the microglia. Genes Dis. 2022, 9, 401–414.
- Das, S.S.; Jha, N.K.; Jha, S.K.; Verma, P.R.P.; Ashraf, G.M.; Singh, S.K. Neuroprotective Role of Quercetin against Alpha-Synuclein-Associated Hallmarks in Parkinson’s Disease. Curr. Neuropharmacol. 2023, 21, 1464–1466.
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59.
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against beta-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219.
- Goyal, A.; Verma, A.; Dubey, N.; Raghav, J.; Agrawal, A. Naringenin: A prospective therapeutic agent for Alzheimer’s and Parkinson’s disease. J. Food Biochem. 2022, 46, e14415.
- Fatima, U.; Roy, S.; Ahmad, S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Islam, A.; Hassan, M.I. Investigating neuroprotective roles of Bacopa monnieri extracts: Mechanistic insights and therapeutic implications. Biomed. Pharmacother. 2022, 153, 113469.
- Li, J.; Zhao, R.; Jiang, Y.; Xu, Y.; Zhao, H.; Lyu, X.; Wu, T. Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct. 2020, 11, 1572–1584.
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARgamma and Abeta42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148.
- Rahnasto-Rilla, M.K.; McLoughlin, P.; Kulikowicz, T.; Doyle, M.; Bohr, V.A.; Lahtela-Kakkonen, M.; Ferrucci, L.; Hayes, M.; Moaddel, R. The Identification of a SIRT6 Activator from Brown Algae Fucus distichus. Mar. Drugs 2017, 15, 190.
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M.; et al. Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8918914.
- Wang, Y.; Wang, Q.; Duan, L.; Li, X.; Yang, W.; Huang, T.; Kong, M.; Guan, F.; Ma, S. Fucoidan ameliorates LPS-induced neuronal cell damage and cognitive impairment in mice. Int. J. Biol. Macromol. 2022, 222, 759–771.
- Kim, J.; Lee, K.P.; Beak, S.; Kang, H.R.; Kim, Y.K.; Lim, K. Effect of black chokeberry on skeletal muscle damage and neuronal cell death. J. Exerc. Nutr. Biochem. 2019, 23, 26–31.
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016.
- Yuan, J.; Zou, M.; Xiang, X.; Zhu, H.; Chu, W.; Liu, W.; Chen, F.; Lin, J. Curcumin improves neural function after spinal cord injury by the joint inhibition of the intracellular and extracellular components of glial scar. J. Surg. Res. 2015, 195, 235–245.
- Abd El-Aal, S.A.; AbdElrahman, M.; Reda, A.M.; Afify, H.; Ragab, G.M.; El-Gazar, A.A.; Ibrahim, S.S.A. Galangin mitigates DOX-induced cognitive impairment in rats: Implication of NOX-1/Nrf-2/HMGB1/TLR4 and TNF-alpha/MAPKs/RIPK/MLKL/BDNF. Neurotoxicology 2022, 92, 77–90.
- Li, S.; Hong, Z.; Wang, Z.; Li, F.; Mei, J.; Huang, L.; Lou, X.; Zhao, S.; Song, L.; Chen, W.; et al. The Cyclopeptide Astin C Specifically Inhibits the Innate Immune CDN Sensor STING. Cell Rep. 2018, 25, 3405–3421.E7.
- Balez, R.; Steiner, N.; Engel, M.; Munoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450.
- de la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430.
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z.; et al. (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 49, 1350–1363.
- Kim, H.G.; Ju, M.S.; Ha, S.K.; Lee, H.; Lee, H.; Kim, S.Y.; Oh, M.S. Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Biol. Pharm. Bull. 2012, 35, 1287–1294.
- Ha, S.K.; Moon, E.; Lee, P.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. Acacetin attenuates neuroinflammation via regulation the response to LPS stimuli in vitro and in vivo. Neurochem. Res. 2012, 37, 1560–1567.
- Li, Y.; Lu, J.; Hou, Y.; Huang, S.; Pei, G. Alzheimer’s Amyloid-beta Accelerates Human Neuronal Cell Senescence Which Could Be Rescued by Sirtuin-1 and Aspirin. Front. Cell. Neurosci. 2022, 16, 906270.
- Anekonda, T.S.; Wadsworth, T.L.; Sabin, R.; Frahler, K.; Harris, C.; Petriko, B.; Ralle, M.; Woltjer, R.; Quinn, J.F. Phytic acid as a potential treatment for alzheimer’s pathology: Evidence from animal and in vitro models. J. Alzheimer’s Dis. 2011, 23, 21–35.
- Ibrayeva, A.; Bay, M.; Pu, E.; Jorg, D.J.; Peng, L.; Jun, H.; Zhang, N.; Aaron, D.; Lin, C.; Resler, G.; et al. Early stem cell aging in the mature brain. Cell Stem Cell 2021, 28, 955–966.e7.
- Mahmoudi, R.; Ghareghani, M.; Zibara, K.; Tajali Ardakani, M.; Jand, Y.; Azari, H.; Nikbakht, J.; Ghanbari, A. Alyssum homolocarpum seed oil (AHSO), containing natural alpha linolenic acid, stearic acid, myristic acid and beta-sitosterol, increases proliferation and differentiation of neural stem cells in vitro. BMC Complement. Altern. Med. 2019, 19, 113.
- Jiang, L.H.; Yang, N.Y.; Yuan, X.L.; Zou, Y.J.; Zhao, F.M.; Chen, J.P.; Wang, M.Y.; Lu, D.X. Daucosterol promotes the proliferation of neural stem cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 90–99.
- Hamedi, A.; Ghanbari, A.; Razavipour, R.; Saeidi, V.; Zarshenas, M.M.; Sohrabpour, M.; Azari, H. Alyssum homolocarpum seeds: Phytochemical analysis and effects of the seed oil on neural stem cell proliferation and differentiation. J. Nat. Med. 2015, 69, 387–396.
- Kong, S.Y.; Park, M.H.; Lee, M.; Kim, J.O.; Lee, H.R.; Han, B.W.; Svendsen, C.N.; Sung, S.H.; Kim, H.J. Kuwanon V inhibits proliferation, promotes cell survival and increases neurogenesis of neural stem cells. PLoS ONE 2015, 10, e0118188.
- Li, Y.J.; Li, Y.J.; Yang, L.D.; Zhang, K.; Zheng, K.Y.; Wei, X.M.; Yang, Q.; Niu, W.M.; Zhao, M.G.; Wu, Y.M. Silibinin exerts antidepressant effects by improving neurogenesis through BDNF/TrkB pathway. Behav. Brain Res. 2018, 348, 184–191.
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654.
- Tiwari, S.K.; Agarwal, S.; Tripathi, A.; Chaturvedi, R.K. Bisphenol-A Mediated Inhibition of Hippocampal Neurogenesis Attenuated by Curcumin via Canonical Wnt Pathway. Mol. Neurobiol. 2016, 53, 3010–3029.
- Wang, J.L.; Wang, J.J.; Cai, Z.N.; Xu, C.J. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62. Int. J. Mol. Med. 2018, 42, 2481–2488.
- Lee, J.G.; Yon, J.M.; Lin, C.; Jung, A.Y.; Jung, K.Y.; Nam, S.Y. Combined treatment with capsaicin and resveratrol enhances neuroprotection against glutamate-induced toxicity in mouse cerebral cortical neurons. Food Chem. Toxicol. 2012, 50, 3877–3885.
- Effros, R.B. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Exp. Gerontol. 2011, 46, 135–140.
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507.
- Tran, H.T.T.; Schreiner, M.; Schlotz, N.; Lamy, E. Short-Term Dietary Intervention with Cooked but Not Raw Brassica Leafy Vegetables Increases Telomerase Activity in CD8+ Lymphocytes in a Randomized Human Trial. Nutrients 2019, 11, 786.
- Berezutsky, M.A.; Durnova, N.A.; Vlasova, I.A. Experimental and clinical studies of mechanisms of the anti-aging effects of chemical compounds in Astragalus membranaceus (review). Adv. Gerontol. 2019, 32, 702–710.
- Radwan, R.A.; El-Sherif, Y.A.; Salama, M.M. A Novel Biochemical Study of Anti-Ageing Potential of Eucalyptus Camaldulensis Bark Waste Standardized Extract and Silver Nanoparticles. Colloids Surfaces B Biointerfaces 2020, 191, 111004.
- Refaey, M.S.; Abdelhamid, R.A.; Elimam, H.; Elshaier, Y.; Ali, A.A.; Orabi, M.A.A. Bioactive constituents from Thunbergia erecta as potential anticholinesterase and anti-ageing agents: Experimental and in silico studies. Bioorg. Chem. 2021, 108, 104643.
- Yan, S.; Lin, S.; Chen, K.; Yin, S.; Peng, H.; Cai, N.; Ma, W.; Songyang, Z.; Huang, Y. Natural Product Library Screens Identify Sanguinarine Chloride as a Potent Inhibitor of Telomerase Expression and Activity. Cells 2022, 11, 1485.
- Saretzki, G. The Telomerase Connection of the Brain and Its Implications for Neurodegenerative Diseases. Stem Cells 2023, 41, 233–241.
- Shibu, M.A.; Lin, Y.J.; Chiang, C.Y.; Lu, C.Y.; Goswami, D.; Sundhar, N.; Agarwal, S.; Islam, M.N.; Lin, P.Y.; Lin, S.Z.; et al. Novel anti-aging herbal formulation Jing Si displays pleiotropic effects against aging associated disorders. Biomed. Pharmacother. 2022, 146, 112427.
- Progatzky, F.; Shapiro, M.; Chng, S.H.; Garcia-Cassani, B.; Classon, C.H.; Sevgi, S.; Laddach, A.; Bon-Frauches, A.C.; Lasrado, R.; Rahim, M.; et al. Regulation of intestinal immunity and tissue repair by enteric glia. Nature 2021, 599, 125–130.
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19.
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537.
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129.
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, M.S.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, M.A.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247.
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140.
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358.
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Qin, N.; Chen, S.D.; Xiao, Q. Detection of Microbial 16S rRNA Gene in the Blood of Patients with Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 156.
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheime’rs Dis. 2021, 81, 583–595.
- Yildirim, S.; Nalbantoglu, O.U.; Bayraktar, A.; Ercan, F.B.; Gundogdu, A.; Velioglu, H.A.; Gol, M.F.; Soylu, A.E.; Koc, F.; Gulpinar, E.A.; et al. Stratification of the Gut Microbiota Composition Landscape across the Alzheimer’s Disease Continuum in a Turkish Cohort. mSystems 2022, 7, e0000422.
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407.
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360.
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164.
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737.
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202.
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405.
- Dodiya, H.B.; Lutz, H.L.; Weigle, I.Q.; Patel, P.; Michalkiewicz, J.; Roman-Santiago, C.J.; Zhang, C.M.; Liang, Y.; Srinath, A.; Zhang, X.; et al. Gut microbiota-driven brain Abeta amyloidosis in mice requires microglia. J. Exp. Med. 2022, 219, e20200895.
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res. 2020, 64, e1900636.
- Lekchand Dasriya, V.; Samtiya, M.; Dhewa, T.; Puniya, M.; Kumar, S.; Ranveer, S.; Chaudhary, V.; Vij, S.; Behare, P.; Singh, N.; et al. Etiology and management of Alzheimer’s disease: Potential role of gut microbiota modulation with probiotics supplementation. J. Food Biochem. 2022, 46, e14043.
- Vaiserman, A.; Koliada, A.; Lushchak, O. Neuroinflammation in pathogenesis of Alzheimer’s disease: Phytochemicals as potential therapeutics. Mech. Ageing Dev. 2020, 189, 111259.
- Wang, Y.; Lim, Y.Y.; He, Z.; Wong, W.T.; Lai, W.F. Dietary phytochemicals that influence gut microbiota: Roles and actions as anti-Alzheimer agents. Crit. Rev. Food Sci. Nutr. 2022, 62, 5140–5166.
- El Gaamouch, F.; Chen, F.; Ho, L.; Lin, H.Y.; Yuan, C.; Wong, J.; Wang, J. Benefits of dietary polyphenols in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1019942.
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40.
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J. Neurol. Sci. 2022, 434, 120166.
- Xiong, W.; Zhao, X.; Xu, Q.; Wei, G.; Zhang, L.; Fan, Y.; Wen, L.; Liu, Y.; Zhang, T.; Zhang, L.; et al. Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 2022, 282, 114598.
- Sun, Y.; Liu, Z.; Pi, Z.; Song, F.; Wu, J.; Liu, S. Poria cocos could ameliorate cognitive dysfunction in APP/PS1 mice by restoring imbalance of Abeta production and clearance and gut microbiota dysbiosis. Phytother. Res. 2021, 35, 2678–2690.
- Terzo, S.; Amato, A.; Mule, F. From obesity to Alzheimer’s disease through insulin resistance. J. Diabetes Complicat. 2021, 35, 108026.
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766.
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258.
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722–2740.
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329.
- Si, H.; Lai, C.Q.; Liu, D. Dietary Epicatechin, A Novel Anti-aging Bioactive Small Molecule. Curr. Med. Chem. 2021, 28, 3–18.
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191.
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160.
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359.
- Hill, M.A.; Gammie, S.C. Alzheimer’s disease large-scale gene expression portrait identifies exercise as the top theoretical treatment. Sci. Rep. 2022, 12, 17189.
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532.
- Grubman, A.; Chew, G.; Ouyang, J.F.; Sun, G.; Choo, X.Y.; McLean, C.; Simmons, R.K.; Buckberry, S.; Vargas-Landin, D.B.; Poppe, D.; et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 2019, 22, 2087–2097.
- Hajdarovic, K.H.; Yu, D.; Hassell, L.A.; Evans, S.; Packer, S.; Neretti, N.; Webb, A.E. Single-cell analysis of the aging female mouse hypothalamus. Nat. Aging 2022, 2, 662–678.
- Yan, X.W.; Liu, H.J.; Hong, Y.X.; Meng, T.; Du, J.; Chang, C. lncRNA XIST induces Abeta accumulation and neuroinflammation by the epigenetic repression of NEP in Alzheimer’s disease. J. Neurogenet. 2022, 36, 11–20.
- Li, Y.; Yuan, X.; Shi, Z.; Wang, H.; Ren, D.; Zhang, Y.; Fan, Y.; Liu, Y.; Cui, Z. LncRNA XIST serves as a diagnostic biomarker in gestational diabetes mellitus and its regulatory effect on trophoblast cell via miR-497-5p/FOXO1 axis. Cardiovasc. Diagn. Ther. 2021, 11, 716–725.
- Pineda, J.R.; Daynac, M.; Chicheportiche, A.; Cebrian-Silla, A.; Sii Felice, K.; Garcia-Verdugo, J.M.; Boussin, F.D.; Mouthon, M.A. Vascular-derived TGF-beta increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol. Med. 2013, 5, 548–562.
- Xiang, Q.; Tian, F.; Xu, J.; Du, X.; Zhang, S.; Liu, L. New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1844–1867.
- Propson, N.E.; Roy, E.R.; Litvinchuk, A.; Kohl, J.; Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J. Clin. Investig. 2021, 131, e144348.
- Peddakkulappagari, C.S.; Saifi, M.A.; Khurana, A.; Anchi, P.; Singh, M.; Godugu, C. Withaferin A ameliorates renal injury due to its potent effect on inflammatory signaling. Biofactors 2019, 45, 750–762.
- Wang, Z.; Hou, X.; Li, M.; Ji, R.; Li, Z.; Wang, Y.; Guo, Y.; Liu, D.; Huang, B.; Du, H. Active fractions of golden-flowered tea (Camellia nitidissima Chi) inhibit epidermal growth factor receptor mutated non-small cell lung cancer via multiple pathways and targets in vitro and in vivo. Front. Nutr. 2022, 9, 1014414.
