Antimicrobial resistance (AMR) due to the prevalence of multidrug-resistant (MDR) pathogens is rapidly increasing worldwide, and the identification of new antimicrobial agents with innovative mechanisms of action is urgently required. Medicinal plants that have been utilised for centuries with minor side effects may hold great promise as sources of effective antimicrobial products. The free-living nematode Caenorhabditis elegans (C. elegans) is an excellent live infection model for the discovery and development of new antimicrobial compounds. However, while C. elegans has widely been utilised to explore the effectiveness and toxicity of synthetic antibiotics, it has not been used to a comparable extent for the analysis of natural products.
- natural products
- antimicrobial activity
- multidrug-resistant pathogens
1. Advantages of Caenorhabditis elegans as a Model for the Screening of Novel Compounds
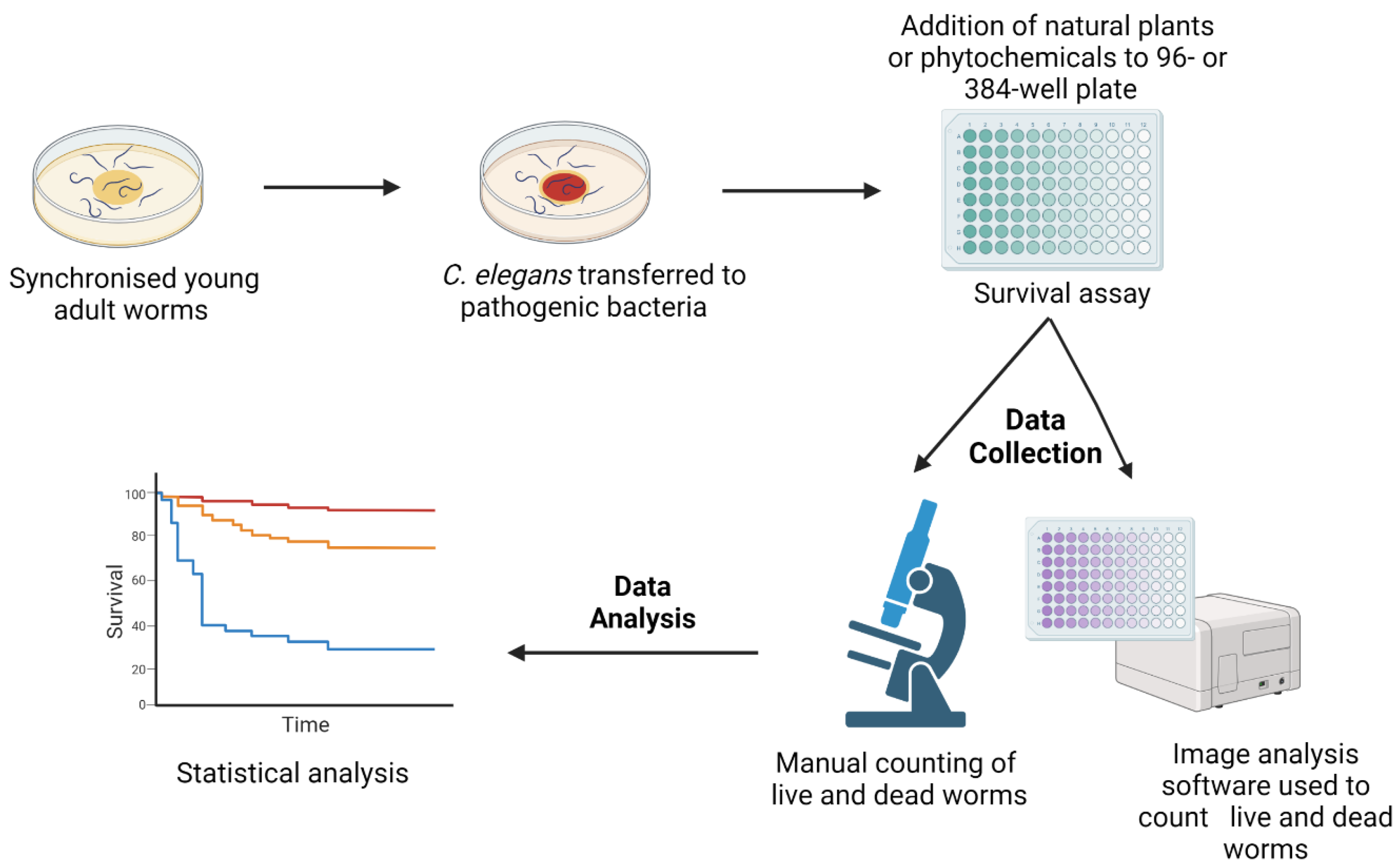
2. Natural Products Active against Bacterial Infection
3. Natural Products Active against Fungal Infection
This entry is adapted from the peer-reviewed paper 10.3390/ph16081070
References
- Moy, T.I.; Ball, A.R.; Anklesaria, Z.; Casadei, G.; Lewis, K.; Ausubel, F.M. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl. Acad. Sci. USA 2006, 103, 10414–10419.
- Moy, T.I.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; Mazitschek, R.; Casadei, G.; Lewis, K.; Carpenter, A.E.; Ausubel, F.M. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009, 4, 527–533.
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109.
- Adonizio, A.; Leal, S.M.; Ausubel, F.M.; Mathee, K. Attenuation of Pseudomonas aeruginosa virulence by medicinal plants in a Caenorhabditis elegans model system. J. Med. Microbiol. 2008, 57, 809–813.
- Burns, A.R.; Kwok, T.C.; Howard, A.; Houston, E.; Johanson, K.; Chan, A.; Cutler, S.R.; McCourt, P.; Roy, P.J. High-throughput screening of small molecules for bioactivity and target identification in Caenorhabditis elegans. Nat. Protoc. 2006, 1, 1906–1914.
- Tampakakis, E.; Okoli, I.; Mylonakis, E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 2008, 3, 1925–1931.
- Gosai, S.J.; Kwak, J.H.; Luke, C.J.; Long, O.S.; King, D.E.; Kovatch, K.J.; Johnston, P.A.; Shun, T.Y.; Lazo, J.S.; Perlmutter, D.H.; et al. Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin alpha1-antitrypsin Z. PLoS ONE 2010, 5, e15460.
- Giunti, S.; Andersen, N.; Rayes, D.; De Rosa, M.J. Drug discovery: Insights from the invertebrate Caenorhabditis elegans. Pharmacol. Res. Perspect. 2021, 9, e00721.
- Peterson, N.D.; Pukkila-Worley, R. Caenorhabditis elegans in high-throughput screens for anti-infective compounds. Curr. Opin. Immunol. 2018, 54, 59–65.
- Phillips, P.C. Self-fertilization sweeps up variation in the worm genome. Nat. Genet. 2012, 44, 237–238.
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, 10–1128.
- Kong, C.; Yehye, W.A.; Abd Rahman, N.; Tan, M.W.; Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement Altern. Med. 2014, 14, 4.
- Patel, P.; Joshi, C.; Kothari, V. Anti-Pathogenic Efficacy and Molecular Targets of a Polyherbal Wound- Care Formulation (Herboheal) against Staphylococcus aureus. Infect. Disord. Drug Targets 2019, 19, 193–206.
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680.
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192.
- Zhou, Y.M.; Shao, L.; Li, J.A.; Han, L.Z.; Cai, W.J.; Zhu, C.B.; Chen, D.J. An efficient and novel screening model for assessing the bioactivity of extracts against multidrug-resistant Pseudomonas aeruginosa using Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2011, 75, 1746–1751.
- Zhang, Y.; Mi, D.Y.; Wang, J.; Luo, Y.P.; Yang, X.; Dong, S.; Ma, X.M.; Dong, K.Z. Constituent and effects of polysaccharides isolated from Sophora moorcroftiana seeds on lifespan, reproduction, stress resistance, and antimicrobial capacity in Caenorhabditis elegans. Chin. J. Nat. Med. 2018, 16, 252–260.
- Dharmalingam, K.; Tan, B.K.; Mahmud, M.Z.; Sedek, S.A.; Majid, M.I.; Kuah, M.K.; Sulaiman, S.F.; Ooi, K.L.; Khan, N.A.; Muhammad, T.S.; et al. Swietenia macrophylla extract promotes the ability of Caenorhabditis elegans to survive Pseudomonas aeruginosa infection. J. Ethnopharmacol. 2012, 139, 657–663.
- Evans, E.A.; Kawli, T.; Tan, M.W. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 2008, 4, e1000175.
- Haripriyan, J.; Omanakuttan, A.; Menon, N.D.; Vanuopadath, M.; Nair, S.S.; Corriden, R.; Nair, B.G.; Nizet, V.; Kumar, G.B. Clove Bud Oil Modulates Pathogenicity Phenotypes of the Opportunistic Human Pathogen Pseudomonas aeruginosa. Sci. Rep. 2018, 8, 3437.
- Husain, F.M.; Ahmad, I.; Asif, M.; Tahseen, Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013, 38, 835–844.
- Ganesh, P.S.; Rai, R.V. Inhibition of quorum-sensing-controlled virulence factors of Pseudomonas aeruginosa by Murraya koenigii essential oil: A study in a Caenorhabditis elegans infectious model. J. Med. Microbiol. 2016, 65, 1528–1535.
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597.
- Liu, J.; Hafting, J.; Critchley, A.T.; Banskota, A.H.; Prithiviraj, B. Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegans to Pseudomonas aeruginosa through the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 2013, 79, 7343–7350.
- Kandasamy, S.; Khan, W.; Evans, F.; Critchley, A.T.; Prithiviraj, B. Tasco(R): A product of Ascophyllum nodosum enhances immune response of Caenorhabditis elegans against Pseudomonas aeruginosa infection. Mar. Drugs 2012, 10, 84–105.
- Bag, A.; Bhattacharyya, S.K.; Pal, N.K. Antibacterial potential of hydroalcoholic extracts of triphala components against multidrug-resistant uropathogenic bacteria—A preliminary report. Indian J. Exp. Biol. 2013, 51, 709–714.
- Patel, H.; Patel, F.; Jani, V.; Jha, N.; Ansari, A.; Paliwal, B.; Rathod, B.; Patel, D.; Patel, P.; Kothari, V. Anti-pathogenic potential of a classical ayurvedic Triphala formulation. F1000Res 2019, 8, 1126.
- Khushboo, P.S.; Jadhav, V.M.; Kadam, V.J.; Sathe, N.S. Psoralea corylifolia Linn.-“Kushtanashini”. Pharmacogn. Rev. 2010, 4, 69–76.
- Husain, F.M.; Ahmad, I.; Khan, F.I.; Al-Shabib, N.A.; Baig, M.H.; Hussain, A.; Rehman, M.T.; Alajmi, M.F.; Lobb, K.A. Seed Extract of Psoralea corylifolia and Its Constituent Bakuchiol Impairs AHL-Based Quorum Sensing and Biofilm Formation in Food- and Human-Related Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 351.
- Husain, F.M.; Ahmad, I.; Al-Thubiani, A.S.; Abulreesh, H.H.; AlHazza, I.M.; Aqil, F. Leaf Extracts of Mangifera indica L. Inhibit Quorum Sensing—Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front. Microbiol. 2017, 8, 727.
- Kanwal, S.; Vaitla, P. Streptococcus Pyogenes; StatPearls: Treasure Island, FL, USA, 2023.
- Nandu, T.G.; Subramenium, G.A.; Shiburaj, S.; Viszwapriya, D.; Iyer, P.M.; Balamurugan, K.; Rameshkumar, K.B.; Karutha Pandian, S. Fukugiside, a biflavonoid from Garcinia travancorica inhibits biofilm formation of Streptococcus pyogenes and its associated virulence factors. J. Med. Microbiol. 2018, 67, 1391–1401.
- Viszwapriya, D.; Subramenium, G.A.; Prithika, U.; Balamurugan, K.; Pandian, S.K. Betulin inhibits virulence and biofilm of Streptococcus pyogenes by suppressing ropB core regulon, sagA and dltA. Pathog. Dis. 2016, 74, ftw088.
- Alam, S.T.; Hwang, H.; Son, J.D.; Nguyen, U.T.T.; Park, J.S.; Kwon, H.C.; Kwon, J.; Kang, K. Natural photosensitizers from Tripterygium wilfordii and their antimicrobial photodynamic therapeutic effects in a Caenorhabditis elegans model. J. Photochem. Photobiol. B 2021, 218, 112184.
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041.
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim, S.I.; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377.
- Ibáñez-Peinado, D.; Pina-Pérez, C.; García-Carrión, G.; Martínez, A.; Rodrigo, D. In vivo Antimicrobial Activity Assessment of a Cauliflower By-Product Extract Against Salmonella Typhimurium. Front. Sustain. Food Syst. 2020, 4, 8.
- Sanz-Puig, M.; Lazaro, E.; Armero, C.; Alvares, D.; Martinez, A.; Rodrigo, D.S. Typhimurium virulence changes caused by exposure to different non-thermal preservation treatments using C. elegans. Int. J. Food Microbiol. 2017, 262, 49–54.
- Jacobo-Salcedo Mdel, R.; Gonzalez-Espindola, L.A.; Alonso-Castro, A.J.; Gonzalez-Martinez Mdel, R.; Dominguez, F.; Garcia-Carranca, A. Antimicrobial activity and cytotoxic effects of Magnolia dealbata and its active compounds. Nat. Prod. Commun. 2011, 6, 1121–1124.
- Kim, H.-I.; Kim, J.-A.; Choi, E.-J.; Harris, J.B.; Jeong, S.-Y.; Son, S.-J.; Kim, Y.; Shin, O.S. In vitro and in vivo antimicrobial efficacy of natural plant-derived compounds against Vibrio cholerae of O1 El Tor Inaba serotype. Biosci. Biotechnol. Biochem. 2015, 79, 475–483.
- Eng, S.A.; Nathan, S. Curcumin rescues Caenorhabditis elegans from a Burkholderia pseudomallei infection. Front. Microbiol. 2015, 6, 290.
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154.
- Coleman, J.J.; Okoli, I.; Tegos, G.P.; Holson, E.B.; Wagner, F.F.; Hamblin, M.R.; Mylonakis, E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem. Biol. 2010, 5, 321–332.
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474.
- Rosas, E.C.; Correa, L.B.; das Graças Henriques, M. Chapter 28—Antiinflammatory Properties of Schinus terebinthifolius and Its Use in Arthritic Conditions. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 489–505.
- De Paula, E.S.A.C.; Costa-Orlandi, C.B.; Gullo, F.P.; Sangalli-Leite, F.; de Oliveira, H.C.; da Silva Jde, F.; Scorzoni, L.; Pitangui Nde, S.; Rossi, S.A.; Benaducci, T.; et al. Antifungal Activity of Decyl Gallate against Several Species of Pathogenic Fungi. Evid. Based Complement. Altern. Med. 2014, 2014, 506273.
- Singulani, J.L.; Scorzoni, L.; Gomes, P.C.; Nazare, A.C.; Polaquini, C.R.; Regasini, L.O.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Activity of gallic acid and its ester derivatives in Caenorhabditis elegans and zebrafish (Danio rerio) models. Future Med. Chem. 2017, 9, 1863–1872.
- Shu, C.; Sun, L.; Zhang, W. Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immunol. Res. 2016, 64, 1013–1024.
- Breger, J.; Fuchs, B.B.; Aperis, G.; Moy, T.I.; Ausubel, F.M.; Mylonakis, E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007, 3, e18.
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Bin Asad, M.H. Caffeic acid phenethyl ester and therapeutic potentials. Biomed. Res. Int. 2014, 2014, 145342.
- Cragg, G.; Newman, D. Natural products and drug discovery and development: A history of success and continuing promise for the future. Planta Med. 2014, 80, IL1.
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321.
- Sun, L.; Liao, K.; Wang, D. Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695.
- Ahmad Khan, M.S.; Altaf, M.M.; Sajid, M. Chapter 14—Insights of Phyto-Compounds as Antipathogenic Agents: Controlling Strategies for Inhibiting Biofilms and Quorum Sensing in Candida albicans. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 367–389.
- Zhao, L.X.; Li, D.D.; Hu, D.D.; Hu, G.H.; Yan, L.; Wang, Y.; Jiang, Y.Y. Effect of Tetrandrine against Candida albicans Biofilms. PLoS ONE. 2013, 8, e79671.
- Benson, J.M.; Nahata, M.C. Clinical use of systemic antifungal agents. Clin. Pharm. 1988, 7, 424–438.
