Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The citrate industry has a wide range of applications in food, pharmaceutical, and other fields. As a common class of food additives and functional supplements with tremendous development potential and strong core competitiveness, particles with good powder characteristics and functionalization are becoming one of the primary directions in the evolution of citrate into the high-end market.
- citrates
- crystallization method
- crystallizer
1. Introduction
As a common class of food additives and functional supplements [1] in pharmaceuticals [2][3][4], medicine [5][6][7][8][9][10][11], healthcare [12][13], and daily chemicals [14], the market for citrate products has expanded to include North America, South America, Asia Pacific, Europe, Africa, and other regions. Compared with other inorganic salts, citrate shows superior biological compatibility for ionic supplements for human consumption, including sodium [15][16][17], potassium [18][19][20], calcium [21][22][23], magnesium [24][25][26], and zinc [27][28]. In addition, the lead citrate precursor route, which is very famous and important for synthesizing nanostructural lead oxide from spent lead-acid battery paste, is of great significance in the recovery of waste lead-acid batteries [29][30]. Therefore, citrate has tremendously desirable development potential and strong core competitiveness over time [31][32][33][34]. Citrate research and commercial trends have steadily shifted toward enhancing the numerous functional requirements of substances, including those for food, materials, reagents, etc. In terms of product quality, particles with good powder properties and functionalization are becoming one of the primary directions in the evolution of citrate into a high-end supplement [35]. For the purpose of assuring the security and efficacy of direct action on the human body, the market is growing more demanding in terms of quality indicators such purity, particle size, grain shape, and other factors of citrate products. On the other hand, higher requirements are also presented by adsorption, crystallization, granulation, process efficiency, and other post-treatment operations [36].
Citrate is commonly used in the form of multiple citrate hydrates. The majority of citrate prepared currently by reactive crystallization or crystallization methods that combine reaction with cooling or evaporation is needle-like powder of low stack density, poor flowability, and poor suspension stability. Therefore, there is a pressing need to develop an effective regulatory mechanism for the preparation of form-controlled and shape-controlled citrate crystals [37][38][39]. However, precise product quality control through quantifying variables is challenging. Essentially, the difficulty is a result of the complexity of the crystallization, which often entails a number of steps including mixing of the raw materials, reactions, crystal nucleation, growth, agglomeration, and fragmentation. In particular, the pressure of effective control of the entire process has been substantially expanded owing to the setting needs of the cooling procedure and the requirement for modification of the evaporation temperature [35]. In general, the sensible and correct control process of crystallization and purification is an urgent issue that needs to be addressed and is key to the significant improvement of the properties of products.
2. Mechanism of Crystallization of Citrate
2.1. Main Methods for Citrate Crystallization
Crystallization is one of the critical processes for purifying solid compounds. The primary method of crystallization for creating premium citrate products has gained increasing attention with the global trend toward high-end fine chemicals. To ensure the stability of the crystallization process, the choice of crystallization methods is crucial. According to the properties of crystal phase transition, solution crystallization and reactive crystallization, including evaporation crystallization, cooling crystallization, and reactive crystallization, are the main crystallization techniques employed in the manufacturing of citrate. The chemical reaction is the driving force in the reactive crystallization process. To achieve separation and purification, the differences in solubility between the constituents are key factors. Cooling crystallization produces the driving force of crystallization by lowering the temperature, whereas evaporative crystallization produces it by removing solvent. A vacuum level was created by decompression techniques to strengthen the driving power of the processes and encourage the initiation and growth of crystals during the crystallization process [40][41][42][43]. In general, the solubility of the substance can be used to select rapidly and conveniently the appropriate crystallization form.
The recombination reaction-coupled crystallization process is regarded as one of the crystallization methods with great potential because of the substantial solubility differences between zinc citrate, magnesium citrate, and calcium citrate products and reactants. While potassium and sodium citrate have substantial solubilities and their solubilities fluctuate greatly with temperature; the simple reaction crystallization procedure cannot provide enough driving power to encourage the formation of crystals. Therefore, the combination of reactive crystallization with cooling crystallization or evaporative crystallization is a common choice. Researchers have paid considerable attention to the reaction materials in the citrate crystallization system due to their high potential for biocompatibility and environmental friendliness. Especially recently, researchers have become more and more interested in biological calcium sources, because their utilization is expected to lessen environmental stress and address the issue of low use of biological waste shells and bones [44][45][46][47][48][49].
Reaction crystallization is a process that couples reaction and crystallization, and the study of kinetics encompasses both reaction kinetics and crystallization kinetics. The complexity of the study of the coupling process is therefore considerably enhanced. Meanwhile, the multiple processes included in a reaction also make it more challenging to precisely regulate the ensuing crystallization process [35]. The use of computer simulation and machine learning methods could significantly improve the predictability and controllability of crystal growth through online, real-time observation and evaluation of the reactive crystallization process. Furthermore, the effective and precise control of the reactive crystallization process is increasingly anticipated [50][51]. To provide the driving force for crystallization, cooling crystallization necessitates heat exchange with the cooling medium, which lowers the temperature of the solution, and evaporative crystallization needs heat to speed up the evaporation of water molecules. However, these operations use a lot of energy, which remarkably raises the cost of the process. To meet market demand, the development of eco-friendly and effective crystallization purification technology and the renewal of crystallization machinery will raise increasing concerns, with the intention of decreasing energy consumption, shortening the crystallization cycle, and boosting crystallization efficiency during the production of citric acid and citrate [52][53][54][55].
2.2. Formation of Citrate Hydrate
Citrate contains a large number of hydrates, and this is one of the most notable properties of citrate crystallization. Hydrates with different water contents exhibit considerable changes in solubility, dissolution rate, appearance, and bioavailability, affecting crystal stability, bioavailability, and efficacy. In particular, anhydrous citrate is a scarce and highly profitable product around the world. In general, the ability of the procedure to produce a single stable form of crystal is an inevitable requirement for product purity. On the other hand, it also has an indirect impact on the international competitiveness of products.
The two most common hydrates of sodium citrate are dihydrate and pentahydrate. Potassium citrate is mainly monohydrate [56]. In the fields of food processing, daily chemical, and pharmaceuticals, raw materials such as sodium citrate and potassium citrate products are required to be water-free. The transition from hydrate to anhydrous geocrystalline form can be achieved simply by adjusting the drying temperature [57][58][59].
Dihydrate and trihydrate are two forms of zinc citrate hydrates. Since 2015, the production process of dihydrate has been continuously developed in order to increase the content of zinc citrate in the product [60]. Moreover, for the purpose of enhancing particle uniformity and the purity of zinc citrate dihydrate crystal products, good control of the reaction system conditions, especially pH, is critical. Some studies report that when the pH of the reaction end point is kept within the range of 4.5–5.5, the content of zinc citrate in the manufactured products can reach 97.0–99.8% by weight [60][61].
The forms of calcium citrate hydrate consist of monohydrate, dihydrate, trihydrate, tetrahydrate, and hexahydrate. The characteristics between different calcium citrate forms are varied, and are usually impacted significantly by temperature. For example, hexahydrate crystallizes at low temperatures, and tetrahydrate crystallizes at higher temperatures. The conversion point between these is 51.6 °C [62][63]. Furthermore, anhydrous matter and dihydrate crystallize to tetrahydrate via solvo-mediated transformation, and the tetrahydrate dehydrates into protocrystalline form via one-step dehydration at 80 °C and two-step dehydration at 130 °C [64][65].
Magnesium citrate is mainly nonahydrate. According to extensive research on the pyrolysis mechanism and microstructure evolution throughout the pyrolysis process, anhydrous products can be obtained with a drying temperature of 150 °C and a breakdown point of about 300 °C. However, the drying temperature of magnesium nonahydrate is only around 70–80 °C [66][67]. Terahertz (THz) time-domain spectroscopy can be used as an effective means to detect and analyze various citrates and distinguish their crystalline hydrates. Researchers discovered that the distinctive terahertz absorption peaks were significantly affected by water content and crystalline state of the metal cation species of a citrate sample [68]. Additionally, variations in temperature, pressure, and solvent may cause the adsorption and removal of solvent water in hydrates, resulting in the crystalline transition of hydrates and subsequent loss of product purity. Further study is needed in the field of stabilizing the production process to create products with a particular water content.
Trihydrate is the common hydrate form of lead citrate. Research has shown that the thermal decomposition process can be divided into three stages: dewatering at 100–200 °C, organic constituent decomposition, and, subsequently, burning. Furthermore, the decomposition of lead citrate in air is significantly affected by roasting temperature. The main components of the product with low-temperature roasting are α-PbO, β-PbO and metallic lead, while the main components of the product with high-temperature roasting are β-PbO [69].
2.3. Nucleation and Growth of Citrate
The fundamental process of crystallization involves crystal nucleation and growth, which determine the structure, form, and characteristics of the crystal, and the improvement of mechanisms for crystal nucleation and growth has been the driving force behind the development of the crystallization industry for decades [70][71]. Numerous elements influence the formation and growth of the citrate crystal. Therefore, comprehension of citrate nucleation and growth mechanisms is essential to targeting a method for controlling crystal quality and improving the poor crystal shape (e.g., needle-like shape, sheet-like shape, etc.) of citrate products in the current industry. However, systematic research on the mechanism of nucleation and growth of citrate is rare at present. The primary research direction has been toward understanding the effects of solvent ratio, temperature, supersaturation, and other external strengthening methods on crystal nucleation and growth, generally focusing on research at a phenomenal level and essentially boosting nucleation and growth rates.
The usual crystallization methods of citrate involve the interaction of evaporation, cooling, chemical reactions, and crystallization. This synergistic impact gives the nucleation and growth of crystals corresponding properties [72][73]. The supersaturation of evaporative crystallization is the result of solvent removal and forms a gradient of concentration within the droplet, which is a quantitative representation of the driving force during the crystallization process and a crucial reference for determining the timing of crystal seed addition. The effect of supersaturation on the rate of nucleation growth during potassium citrate production was investigated by Luo Hu et al., who discovered that nucleation and crystal growth rates were optimal when crystalline seeds were added and crystallized by evaporation at a degree of supersaturation in the range 1.05–1.15 (the ratio of the weight of solute actually dissolved in the solvent at a given temperature to the weight of solute theoretically dissolved in the solvent when the solution is saturated at that temperature) [74]. Compared with other crystallization methods, reactive crystallization is unique in that the reaction rate affects the time required for the crystallization process, which is a qualitative expression of the costs and benefits in engineering practice. Strengthening the reaction process naturally increases the crystallization rate [75]. Figure 1 illustrates the common nucleation growth pathway of a crystal, standard designs of ultrasound reactors, and representations of different designs for microwave reactors. Over the years, the use of external force fields such as ultrasonic and microwave has become an extremely effective strengthening method that can significantly affect the nucleation growth process. Shi Zhiyong et al. found that the ultrasonic microwave technique is advantageous as it can be used to speed up the crystallization of magnesium citrate. Essentially, an ultrasonic wave can speed up the pace at which the magnesium source dissolves and, in the meantime, encourage the reaction between magnesium source and citric acid through the cavitation effect, and microwaves can hasten the nucleation of crystals while simultaneously speeding up the collision of citric acid and magnesium source molecules in aqueous solution by causing molecular motion through vibration [75][76]. On the other hand, microwave irradiation can also heat the solution uniformly while at the same time supplying the necessary energy to facilitate the quick nucleation and development of crystals. Compared with no ultrasonic microwave process, ultrasonic microwave technology showed significant enhancements in shortening the reaction time and accelerating crystallization. Finally, the nucleation induction time was reduced from 3–4 h to 6–10 min [77]. Furthermore, Li Junfeng et al. discovered that calcium citrate crystal nuclei could form quickly and uniformly under homogeneous water and alcohol mixing conditions because calcium citrate has poor solubility; it is barely soluble in water and insoluble in alcohol, and further growth of crystal nuclei could be inhibited. Direct preparation of nano-sized calcium citrate crystals with a width of about 60 to 700 nm and a thickness of about 20 to 50 nm without the need of crushing or further procedures is possible under these conditions [78].
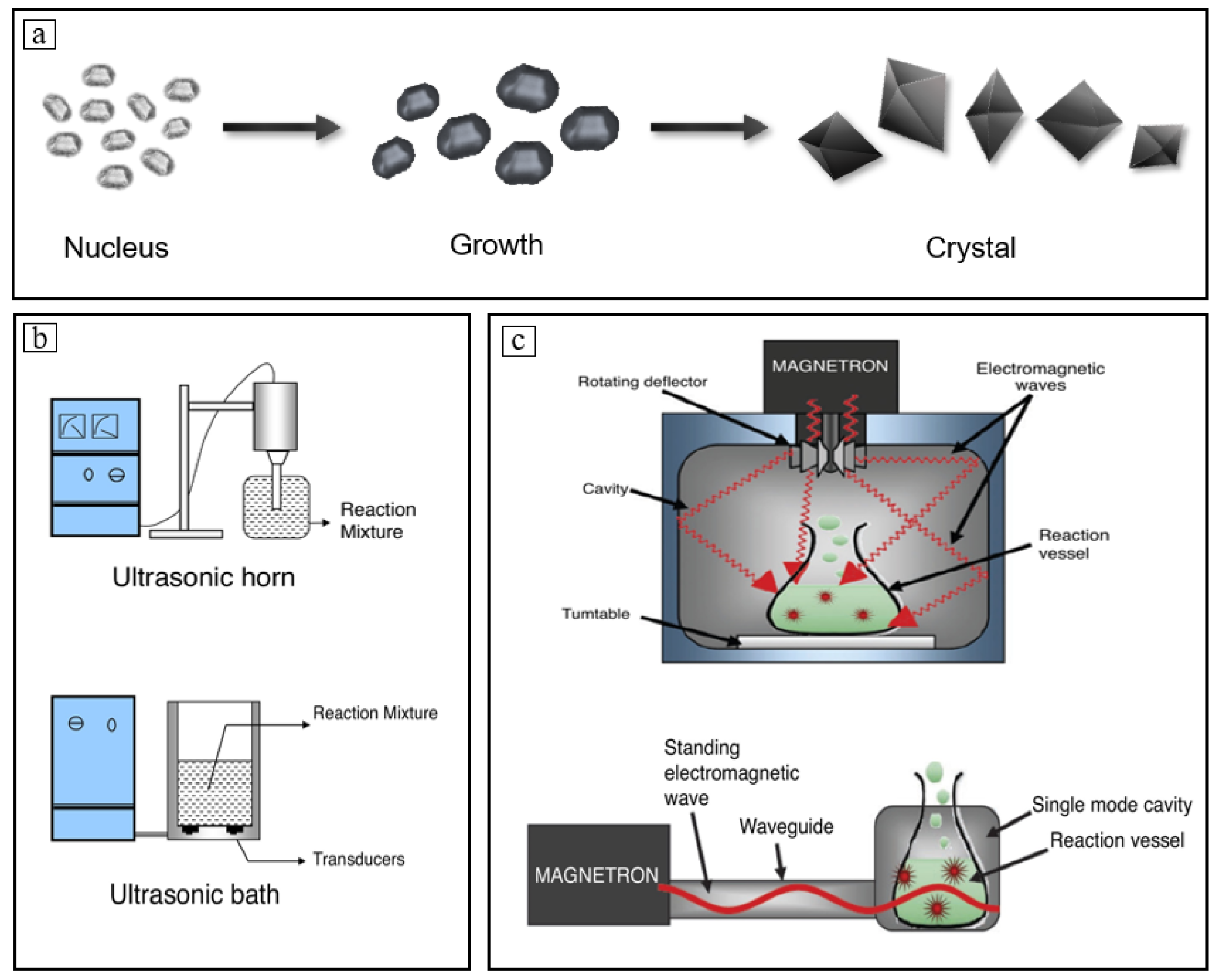
Figure 1. (a) Common nucleation growth pathway of a crystal; (b) standard designs of the ultrasound reactors; (c) representations of different designs for microwave reactors.
In addition to the aforementioned customary investigations on nucleation and growth that concentrated on increasing production efficiency, Yan hypothesized a unique mechanism of noncrystallographic branching during reactive crystallization of calcium citrate, as shown in Figure 2a. The morphological evolution of calcium citrate spherulite can be synoptically divided into three phases according to images of optical microscopy displayed in Figure 2b. In the first phase, the early precursor materials gather to form rod-like crystals that grow from the center to the ends. The beginning of the second phase consists of the front of both sides of the crystal gradually developing small angular branching without clear direction due to the various growth angles. The third phase involves the formation of spherical crystals through the complete filling of the crystal from the center outward in all directions. Additionally, the image shown in Figure 2c indicates that supersaturation and spherulite development are tightly connected [35].
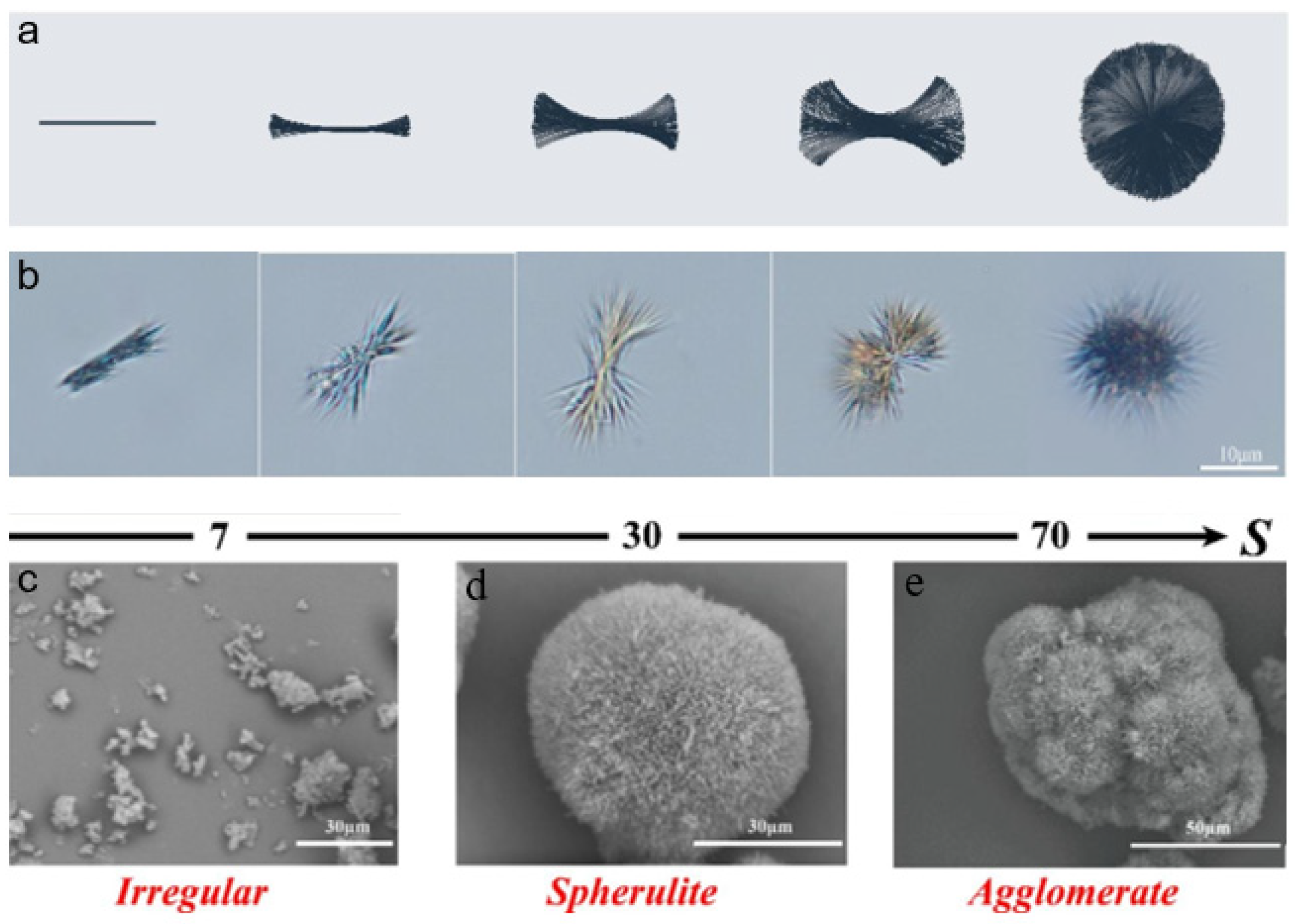
Figure 2. (a) Schematic diagram of the spherulitic growth mechanism; (b) optical microscopy images at different growth stages under the experimental conditions (S = 20, temperature = 40 °C); SEM images at different supersaturation levels: (c) S = 7; (d) S = 30; (e) S = 70.
3. Key Product Indicators and Crystallization Control Measures for Citrate Crystals
3.1. Crystal Purity
As shown in Figure 3, product purity, along with crystal size and grain form, makes up the fundamental quality metrics for citrate products, which have a direct impact on their functionality and potential applications. An effective control approach to address the issue of product purity is to adopt crystal growth condition control, impurity control, post-treatment technology, crystal growth control, crystal structure optimization, etc., based on the specific causes of such difficulties [79][80][81][82][83]. Specifically, the crystal transformation that results in the product becoming a mixture of various crystal forms is primarily responsible for the purity of citrate products. For the purpose of preventing crystal change and increasing product purity, there is an urgent need to ensure a precise operation point and stable operation procedure. On the other hand, in the citrate industry chain, the upstream raw material citric acid is mainly prepared by microbial fermentation; therefore, impurities like mycelium, mineral salts, proteins, and other organic acids will undoubtedly be introduced as a result of the fermentation process, and impurity retention may occur during the growth of citrate crystals, which poses significant challenges relating to the demand for high-purity citrate products [84].
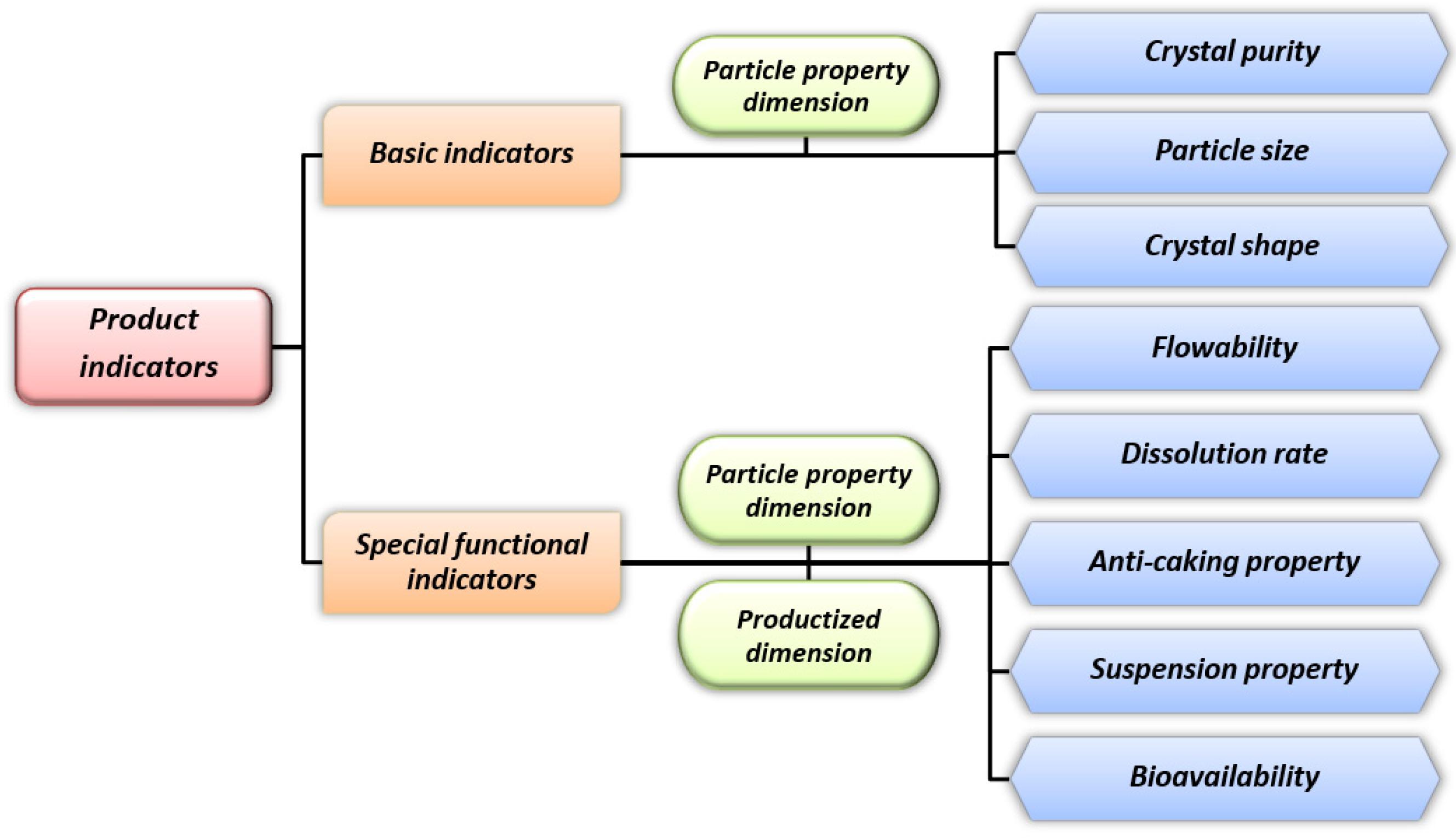
Figure 3. Key product indicators of citrate crystal.
The quality of the raw materials differs significantly between the fermentation process and the chemical method. Recently, the close attention paid to maintaining the purity of raw materials has prompted researchers to respond by improving and developing citric acid purification technology. Some reports have demonstrated that bipolar membrane bioelectrodialysis and two-phase electrodialysis technologies have a wide range of potential applications because of their advantages preventing reverse diffusion brought on by concentration differences and can recover citric acid at higher rates [85][86][87][88]. In addition, another simple and efficient option is to directly buy high-purity raw materials. For example, Wu Jian et al. used high-purity sodium citrate and zinc sulfate heptahydrate as raw materials to prepare zinc citrate, which successfully achieved the goal of effectively controlling the heavy metal content of the product from the reaction source. Furthermore, another primary cause of the decrease in product purity is the inclusion of excessive reactants in the final product [74]. Calcium citrate can be prepared through two-step reactive crystallization, in which calcium sources usually use calcium carbonate and calcium citrate. In a one-step reaction, calcium carbonate reacts with citric acid to form calcium hydrogen citrate, with a reaction endpoint pH of 2.7–3.5. In the second step, calcium hydroxide reacts with calcium citrate to form calcium citrate, and the pH range is 3.8 to 4.5. The excessively high pH means that more alkali sources exist in a reactive system, which may result in the occurrence of raw material encapsulation and thus decrease the purity of the product. Conversely, dissolving loss of the product may increase as a result of too low pH and ultimately lead to a lower yield. Therefore, the aforementioned issues can be greatly reduced by monitoring the pH value of the combination to help establish the ideal ratio of materials and keeping the two-step reaction within the most favorable pH intervals for making calcium citrate [89][90][91][92]. Additionally, the uniformity and stability of the reaction process are crucial for increasing product purity [93][94]. Ultrasonic, microwave, and other applied force fields were used as some of the earliest efficient methods. Yao used alkenyl imidazole salt as the catalytic agent, which generated a microwave reaction environment and ensured that the reaction components made full contact with one another. Finally, this method successfully ensured the uniformity of the reaction, so as to realize the specificity of the product [60][95][96][97].
3.2. Particle Size
Particle size is a crucial aspect of powder characteristics that directly affects the solubility, suspension stability, bioavailability, flowability, and compressibility of the product. Therefore, it has become a necessary indicator to characterize product quality. Product granularity needs vary depending on the functions of products. To achieve precise preparation of products with a specific size distribution, adjusting the crystallization process has become a research target and hotspot [35].
An appropriate degree of supersaturation could regulate the nucleation rate and growth rate of crystals and thus influence the particle size and distribution of products. Hence, it is crucial to adjudicate suitable supersaturation by designing the optimum cooling, cooling rate, and evaporation and concentration temperatures [98]. Based on reported studies, it was discovered that the crystal nucleation and growth rate of sodium citrate were best suited when the cooling rate was adjusted from 5 °C to 12 °C/h, which could then effectively control the particle size of sodium citrate in the range of 0.38~0.83 mm. Furthermore, a common control strategy is to make sure the ion concentration or pH of the reaction material is within the proper range. For example, the particle size distribution of calcium citrate products was best when the concentration of calcium ions was adjusted between 0.001 and 1 mol/L, and the range of pH between 4.1 and 4.5 is superior to others because the zinc citrate crystal particles are more homogeneous within this control zone [94]. Additionally, adding crystal seed is the most effective way to control primary nucleation and induce crystallization. As such, timing and amount of seed addition are crucial factors in determining the final product size [99]. Tang proved that the optimal moment to add the crystal seed during the production process of sodium citrate is when the material and liquid ratio reaches 1.34 g/mL. Meanwhile, maintaining a cooling rate within 5–12 °C/h could finally result in the creation of crystal particles with uniform particle size [98]. In conclusion, based on the research of many scholars, it is apparent that crystal seed plays an absolutely crucial role in optimizing the quality of the final product and controlling the crystallization process. In light of this, it is anticipated that crystal seed addition technology research and development will lead to more effective control of product particle size distribution and improved product quality [100].
This entry is adapted from the peer-reviewed paper 10.3390/cryst13081186
References
- Canales, B.K. Alkalinizing Agents: A Review of Prescription, Over-the-Counter, and Medical Food Supplements. J. Endourol. 2020, 34, 639.
- Hoy, S.M.; Scott, L.J.; Wagstaff, A.J. Sodium picosulfate/magnesium citrate: A review of its use as a colorectal cleanser. Drugs 2009, 69, 123–136.
- Philichi, L.; Yuwono, M. A retrospective study comparing polyethylene glycol-electrolyte solution with magnesium citrate for treatment of fecal disimpaction. Gastroenterol. Nurs. 2018, 41, 141–144.
- Dziechciarz, P.; Ruszczyński, M.; Horvath, A. Sodium Picosulphate with Magnesium Citrate versus Polyethylene Glycol for Bowel Preparation in Children: A Systematic Review. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 228.
- Franklin, R.B.; Costello, L.C. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch. Biochem. Biophys. 2007, 463, 211–217.
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112.
- Zhao, Y.; Liu, X.; Si, F.; Huang, L.; Gao, A.; Lin, W.; Hoft, D.F.; Shao, Q.; Peng, G. Citrate promotes excessive lipid biosynthesis and senescence in tumor cells for tumor therapy. Adv. Sci. 2022, 9, 2101553.
- Castellani, D.; Giulioni, C.; De Stefano, V.; Brocca, C.; Fuligni, D.; Galosi, A.B.; Teoh, J.Y.-C.; Sarica, K.; Gauhar, V. Dietary management of hypocitraturia in children with urolithiasis: Results from a systematic review. World J. Urol. 2023, 41, 1243–1250.
- Spivacow, F.R.; Negri, A.L.; Polonsky, A.; Del Valle, E.E. Long-term treatment of renal lithiasis with potassium citrate. Urology 2010, 76, 1346–1349.
- Doizi, S.; Poindexter, J.R.; Pearle, M.S.; Blanco, F.; Moe, O.W.; Sakhaee, K.; Maalouf, N.M. Impact of potassium citrate vs citric acid on urinary stone risk in calcium phosphate stone formers. J. Urol. 2018, 200, 1278–1284.
- Phillips, R.; Hanchanale, V.S.; Myatt, A.; Somani, B.; Nabi, G.; Biyani, C.S. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst. Rev. 2015, 2015, CD010057.
- Yang, Y.; Li, J.; Wang, L.; Liu, H.; Lai, X. Calcium citrate: An interesting organic calcium biomedical material. Chin. J. Tissue Eng. Res. 2021, 25, 1609.
- Icard, P.; Coquerel, A.; Wu, Z.; Gligorov, J.; Fuks, D.; Fournel, L.; Lincet, H.; Simula, L. Understanding the central role of citrate in the metabolism of cancer cells and tumors: An update. Int. J. Mol. Sci. 2021, 22, 6587.
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks Jr, J.G.; Shank, R.C.; Slaga, T.J. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 2014, 33, 16S–46S.
- Timpmann, S.; Burk, A.; Medijainen, L.; Tamm, M.; Kreegipuu, K.; Vähi, M.; Unt, E.; Ööpik, V. Dietary sodium citrate supplementation enhances rehydration and recovery from rapid body mass loss in trained wrestlers. Appl. Physiol. Nutr. Metab. 2012, 37, 1028–1037.
- Cerullo, G.; Parimbelli, M.; Perna, S.; Pecoraro, M.; Liguori, G.; Negro, M.; D’Antona, G. Sodium citrate supplementation: An updated revision and practical recommendations on exercise performance, hydration status, and potential risks. Transl. Sports Med. 2020, 3, 518–525.
- Redant, S.; De Bels, D.; Massaut, J.; Barglazan, D.; Lebitasy, P.M.; Honoré, P.M. Feasibility of citrate dialysis in hyponatremia: A case series. Blood Purif. 2021, 50, 174–179.
- Moseley, K.F.; Weaver, C.M.; Appel, L.; Sebastian, A.; Sellmeyer, D.E. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J. Bone Miner. Res. 2013, 28, 497–504.
- Nicar, M.J.; Peterson, R.; Pak, C.Y. Use of potassium citrate as potassium supplement during thiazide therapy of calcium nephrolithiasis. J. Urol. 1984, 131, 430–433.
- Carvalho, M.; Erbano, B.O.; Kuwaki, E.Y.; Pontes, H.P.; Liu, J.W.T.W.; Boros, L.H.; Asinelli, M.O.; Baena, C.P. Effect of potassium citrate supplement on stone recurrence before or after lithotripsy: Systematic review and meta-analysis. Urolithiasis 2017, 45, 449–455.
- Palermo, A.; Naciu, A.M.; Tabacco, G.; Manfrini, S.; Trimboli, P.; Vescini, F.; Falchetti, A. Calcium citrate: From biochemistry and physiology to clinical applications. Rev. Endocr. Metab. Disord. 2019, 20, 353–364.
- Gómez, J.M.Q.; Rubió, J.B.; Curiel, M.D.; Pérez, A.D. Calcium citrate and vitamin D in the treatment of osteoporosis. Clin. Drug Investig. 2011, 31, 285–298.
- Tondapu, P.; Provost, D.; Adams-Huet, B.; Sims, T.; Chang, C.; Sakhaee, K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 1256–1261.
- Schutten, J.C.; Joris, P.J.; Mensink, R.P.; Danel, R.M.; Goorman, F.; Heiner-Fokkema, M.R.; Weersma, R.K.; Keyzer, C.A.; de Borst, M.H.; Bakker, S.J. Effects of magnesium citrate, magnesium oxide and magnesium sulfate supplementation on arterial stiffness in healthy overweight individuals: A study protocol for a randomized controlled trial. Trials 2019, 20, 295.
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H. Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial. J. Am. Heart Assoc. 2022, 11, e021783.
- Vermeulen, E.A.; Eelderink, C.; Hoekstra, T.; van Ballegooijen, A.J.; Raijmakers, P.; Beulens, J.W.; de Borst, M.H.; Vervloet, M.G. Reversal Of Arterial Disease by modulating Magnesium and Phosphate (ROADMAP-study): Rationale and design of a randomized controlled trial assessing the effects of magnesium citrate supplementation and phosphate-binding therapy on arterial stiffness in moderate chronic kidney disease. Trials 2022, 23, 769.
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnić, M.; Hurrell, R.F. Zinc Absorption by Young Adults from Supplemental Zinc Citrate Is Comparable with That from Zinc Gluconate and Higher than from Zinc Oxide. J. Nutr. 2014, 144, 132–136.
- Sapota, A.; Daragó, A.; Skrzypińska-Gawrysiak, M.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: A comparative study. Biometals 2014, 27, 495–505.
- Sun, X.; Zhang, W.; Li, H.; Zhu, X.; He, D.; Yang, J. Effects of ultrasound on the crystallization of products prepared from lead paste by hydrometallurgical processes. Chem. Ind. Eng. Prog. 2013, 32, 1974–1978.
- Zhu, X.F.; Liu, W.C.; Yang, H.Y.; Li, L.; Yang, J.K. Preparation of ultrafine PbO powders from lead paste in spent lead acid battery. Trans. Nonferrous Met. Soc. China 2010, 20, 132–136.
- Xiao, Y.; Yang, Y.; Li, J.; Ma, Y.; Wang, H.; Wang, L.; Huang, Y.; Zhang, P.; Zou, Q.; Lai, X. Porous composite calcium citrate/polylactic acid materials with high mineralization activity and biodegradability for bone repair tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 507–520.
- Koc, B.; Kizildag, S.; Hosgorler, F.; Gumus, H.; Kandis, S.; Ates, M.; Uysal, N. Magnesium citrate increases pain threshold and reduces TLR4 concentration in the brain. Biol. Trace Elem. Res. 2021, 199, 1954–1966.
- Anitua, E.; Zalduendo, M.; Troya, M.; Alkhraisat, M.H. The influence of sodium citrate on the characteristics and biological activity of plasma rich in growth factors. Regen. Med. 2020, 15, 2181–2192.
- Ebrahimi, M.; Fakhr Yasseri, A.; Zareian Baghdadabad, L.; Zahmatkesh, P.; Keshavarz Pakseresht, B.; Khoshchehreh, M.; Namazi Shabestari, A. The Impact of Potassium Citrate on the Kidney Stones Treatment in Rat. Transl. Res. Urol. 2021, 3, 176–181.
- Yan, H.; Liu, Y.; Peng, H.; Li, K.; Li, C.; Jiang, S.; Chen, M.; Han, D.; Gong, J. Improving calcium citrate food functions through spherulitic growth in reactive crystallization and a mechanism study. Food Chem. 2023, 404, 134550.
- Sawant, O.; Mahale, S.; Ramchandran, V.; Nagaraj, G.; Bankar, A. Fungal citric acid production using waste materials: A mini-review. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 821–828.
- Luo, H.; Zhou, Y.; Xu, G.; Man, Y.; Wang, J. A Kind of Production Method of Anhydrous Citric Acid Crystal. Patent CN106146292A, 2 April 2015.
- Shi, Z. Preparation Method and Application of Anhydrous Citric Acid. Patent CN114315559A, 10 December 2021.
- Luo, H.; Xiong, J.; Xu, G.; Gao, Z.; Tao, J. A Kind of Method for Preparing Anhydrous Citric Acid Crystal. Patent CN104177251A, 29 July 2014.
- Tippetts, M.; Martini, S. Effect of cooling rate on lipid crystallization in oil-in-water emulsions. Food Res. Int. 2009, 42, 847–855.
- Campos, R.; Narine, S.; Marangoni, A. Effect of cooling rate on the structure and mechanical properties of milk fat and lard. Food Res. Int. 2002, 35, 971–981.
- Cavallo, D.; Gardella, L.; Alfonso, G.C.; Portale, G.; Balzano, L.; Androsch, R. Effect of cooling rate on the crystal/mesophase polymorphism of polyamide 6. Colloid Polym. Sci. 2011, 289, 1073–1079.
- Im, S.H.; Park, O.O. Effect of evaporation temperature on the quality of colloidal crystals at the water−air interface. Langmuir 2002, 18, 9642–9646.
- Aditya, S.; Stephen, J.; Radhakrishnan, M. Utilization of eggshell waste in calcium-fortified foods and other industrial applications: A review. Trends Food Sci. Technol. 2021, 115, 422–432.
- Ding, X.-M.; Peng, L.; Wen, F.; Tan, Z.-W.; Mu, Z.-L. Simulated body fluid immersion method for assessing biological characteristics of calcium citrate. Chin. J. Tissue Eng. Res. 2013, 17, 6811.
- Oliveira, D.; Benelli, P.; Amante, E. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47.
- Ahmed, S.; Gibriel, A.A.; Abdellatif, A.; Ebied, H. Evaluation of food products fortified with oyster shell for the prevention and treatment of osteoporosis. J. Food Sci. Technol. 2015, 52, 6816–6820.
- Zhan, J.; Lu, J.; Wang, D. Review of shell waste reutilization to promote sustainable shellfish aquaculture. Rev. Aquac. 2022, 14, 477–488.
- Xu, Y.; Ye, J.; Zhou, D.; Su, L. Research progress on applications of calcium derived from marine organisms. Sci. Rep. 2020, 10, 18425.
- Salami, H.; McDonald, M.A.; Bommarius, A.S.; Rousseau, R.W.; Grover, M.A. In Situ Imaging Combined with Deep Learning for Crystallization Process Monitoring: Application to Cephalexin Production. Org. Process Res. Dev. 2021, 25, 1670–1679.
- Zheng, Y.; Wu, Z. Predictive Control of Batch Crystallization Process Using Machine Learning. IFAC-PapersOnLine 2022, 55, 798–803.
- Feng, H.; Wang, N.; Huang, X.; Wang, T.; Zhou, L.A.; Hao, H.X. Recent progress in melt crystallization. Chem. Eng. Res. Des. 2023, 190, 268–281.
- Hong, W.; Jia, S.; Chen, Y.; Mou, Q.; Gao, Z.; Gong, J. Highly Efficient and Solvent-Free Purification Technique and Model Study of Layer Melt Crystallization. ACS Sustain. Chem. Eng. 2022, 10, 16450–16458.
- Amran, N.A.; Jusoh, M. Effect of Coolant Temperature and Circulation flowrate on the Performance of a Vertical Finned Crystallizer. In Proceedings of the 4th International Conference on Process Engineering and Advanced Materials (ICPEAM 2016), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 1408–1415.
- Sparenberg, M.-C.; Chergaoui, S.; Sang Sefidi, V.; Luis, P. Crystallization control via membrane distillation-crystallization: A review. Desalination 2021, 519, 115315.
- Xu, H.; Zhang, X. A Kind of Preparation Method of Specific Particle Size Potassium Citrate Monohydrate. Patent CN112010750A, 25 August 2020.
- Shi, Z. The Invention Relates to a Production Method of Anhydrous Sodium Citrate. Patent CN105037140A, 26 June 2015.
- Xu, H.; Zhang, X.; Zhu, Y.; Li, J.; Jia, Q. The Invention Relates to a Preparation Method of Large Granules Anhydrous Sodium Citrate. Patent CN109438225A, 21 November 2018.
- Gao, J. Study on Continuous Crystallization Process of Sodium Citrate Hydrate. Master’s Thesis, Tianjin University, Tianjin, China, 2012.
- Li, B.; Luo, H.; Xiong, J.; Song, J.; Xu, G. A Kind of Zinc Citrate and Production Method Thereof. Patent CN106854147A, 8 December 2015.
- Liu, X.-C.; Skibsted, L.H. Citrate in calcium transport and biomineralisation. Int. Dairy J. 2022, 139, 105561.
- Vavrusova, M.; Skibsted, L.H. Aqueous solubility of calcium citrate and interconversion between the tetrahydrate and the hexahydrate as a balance between endothermic dissolution and exothermic complex formation. Int. Dairy J. 2016, 57, 20–28.
- Liu, X.-C.; Kirkensgaard, J.J.K.; Skibsted, L.H. Hydrates of calcium citrate and their interconversion in relation to calcium bioaccessibility. Food Res. Int. 2021, 140, 109867.
- Qin, Y. Study on Crystallization Process of Citric Acid and Its Calcium Salt. Master’s Thesis, Tianjin University, Tianjin, China, 2014.
- Luo, J.; Xiao, G.; Ding, D.; Chong, X.; Ren, J.; Bai, B. Pyrolysis mechanism of magnesium citrate nonahydrate and microstructural evolution during the process. Ceram. Int. 2021, 47, 29607–29619.
- Li, B.; Zhou, Y.; Guizhen, X.; Song, J. A Kind of Magnesium Citrate Crystal and Production Method Thereof. Patent CN106854145A, 4 September 2020.
- Shen, Y.; Qiao, X.; Song, Z.; Zhong, S.; Wei, D. Terahertz spectroscopy of citrate Salts: Effects of crystalline state and crystallization water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 277, 121288.
- Zhu, X.; Wang, X.; Nie, P.; Zhang, W.; Hu, Y.; Yang, J.; Wang, X.; Guo, Y. Thermal decomposition of lead citrate Pb3(C6H5 O7)2·3H2O from recovery spent lead paste by hydrometallurgy process. Trans. Nonferrous Met. Soc. China 2016, 26, 2686–2693.
- Cölfen, H. Nonclassical Nucleation and Crystallization. Crystals 2020, 10, 61.
- Han, M.; Li, J.; He, G.; Lin, M.; Xiao, W.; Li, X.; Wu, X.; Jiang, X. Tailored 3D printed micro-crystallization chip for versatile and high-efficiency droplet evaporative crystallization. Lab. Chip. 2019, 19, 767–777.
- Zaykovskaya, A.; Louhi-Kultanen, M. Batch Crystallization of Xylitol by Cooling, Evaporative, and Antisolvent Crystallization. Cryst. Growth Des. 2023, 23, 1813–1820.
- Wang, X.; Wu, L.; Wang, G.; Chen, G. Dynamic Crystallization and Phase Transition in Evaporating Colloidal Droplets. Nano Lett. 2019, 19, 8225–8233.
- Luo, H.; Man, Y.; Xu, G.; Wang, J. A Kind of Production Method of Potassium Citrate Crystal and Potassium Citrate Crystal Produced by the Method. Patent CN106854152A, 8 December 2015.
- Gogate, P.R. Intensification of chemical processing applications using ultrasonic and microwave irradiations. Curr. Opin. Chem. Eng. 2017, 17, 9–14.
- Ji, Z.; Wang, J.; Yin, Z.; Hou, D.; Luan, Z. Effect of microwave irradiation on typical inorganic salts crystallization in membrane distillation process. J. Membr. Sci. 2014, 455, 24–30.
- Shi, Z. A Kind of Production Method of Magnesium Citrate. Patent CN114436813A, 4 January 2022.
- Li, J.; Zhong, L.; Gao, Y.; Cao, W.; You, Y.; Zhang, P.; Li, J.; Lai, X. Preparation method of nano calcium citrate. Patent CN103755552A, 24 February 2014.
- Dowling, R.; Davey, R.J.; Curtis, R.A.; Han, G.; Poornachary, S.K.; Chow, P.S.; Tan, R.B. Acceleration of crystal growth rates: An unexpected effect of tailor-made additives. Chem. Commun. 2010, 46, 5924–5926.
- Liu, Y.; Xing, Q.; Dennis, K.W.; McCallum, R.W.; Lograsso, T.A. Evolution of precipitate morphology during heat treatment and its implications for the superconductivity in K x Fe 1.6+ y Se 2 single crystals. Phys. Rev. B 2012, 86, 144507.
- Fan, G.; Li, T.; Zhao, L.; Zhang, S. Study on purification technology of silicon carbide crystal growth powder. Materials 2022, 15, 8190.
- Kwon, S.-J.; Jazbinsek, M.; Kwon, O.-P.; Günter, P. Crystal growth and morphology control of OH1 organic electrooptic crystals. Cryst. Growth Des. 2010, 10, 1552–1558.
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-purity inorganic perovskite films for solar cells with 9.72% efficiency. Angew. Chem. Int. Ed. 2018, 57, 3787–3791.
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76.
- Luo, G.S.; Shan, X.Y.; Qi, X.; Lu, Y.C. Two-phase electro-electrodialysis for recovery and concentration of citric acid. Sep. Purif. Technol. 2004, 38, 265–271.
- Sun, X.; Lu, H.; Wang, J. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. J. Clean. Prod. 2017, 143, 250–256.
- Luo, H.; Cheng, X.; Liu, G.; Zhou, Y.; Lu, Y.; Zhang, R.; Li, X.; Teng, W. Citric acid production using a biological electrodialysis with bipolar membrane. J. Membr. Sci. 2017, 523, 122–128.
- Wang, Q.; Chen, G.Q.; Lin, L.; Li, X.; Kentish, S.E. Purification of organic acids using electrodialysis with bipolar membranes (EDBM) combined with monovalent anion selective membranes. Sep. Purif. Technol. 2021, 279, 119739.
- Wu, J.; Lu, Z.; Tang, Y. The Invention Relates to Water-Soluble Zinc Citrate, Preparation Method and Application Thereof. Patent CN111067106A, 17 December 2019.
- Kou, G.; Li, C.; Liu, C.; Zhou, H.; An, F.; Gao, X. Production Process of Amorphous Ultra-Fine Calcium Citrate. Patent CN104529754A, 24 December 2014.
- Li, C.; Wei, C.; Zhang, H.; Yu, H.; Jiang, S.; Fan, K. The Invention Relates to a Preparation Method of Calcium Citrate Whisker. Patent CN112409167A, 27 November 2020.
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781.
- Kumar, S.G.; Rao, K.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351.
- Yao, J.; Zhu, B.; Yao, J. The Invention Relates to a Synthesis Method of Zinc Citrate Trihydrate. Patent CN104610048A, 11 February 2015.
- Gordon, J.; Kazemian, H.; Rohani, S. Rapid and efficient crystallization of MIL-53 (Fe) by ultrasound and microwave irradiation. Microporous Mesoporous Mater. 2012, 162, 36–43.
- Li, Y.; Huang, H.; Xu, G.; Xiong, J.; Xu, X.; Li, C. The Invention Relates to a Production Method of Zinc Citrate Crystal. Patent CN112174802A, 5 July 2019.
- Ryu, J.H.; Koo, S.-M.; Yoon, J.-W.; Lim, C.S.; Shim, K.B. Synthesis of nanocrystalline MMoO4 (M=Ni, Zn) phosphors via a citrate complex route assisted by microwave irradiation and their photoluminescence. Mater. Lett. 2006, 60, 1702–1705.
- Tang, X.; Nie, Y.; Jin, Q.; Guo, L.; Zhao, J.; Li, T.; Zhu, Y. Kinetics and mechanism of ultrasonic-assisted magnesium oxide hydration. Ultrason. Sonochem. 2018, 40, 995–1002.
- Tang, B.; Peng, L. The Invention Relates to Refining Process and Realization Device of Sodium Citrate with Controllable Particle Size. Patent CN107188798A, 27 May 2017.
- Bergfors, T. Seeds to crystals. J. Struct. Biol. 2003, 142, 66–76.
- Zhang, F.; Shan, B.; Wang, Y.; Zhu, Z.; Yu, Z.-Q.; Ma, C.Y. Progress and opportunities for utilizing seeding techniques in crystallization processes. Org. Process Res. Dev. 2021, 25, 1496–1511.
This entry is offline, you can click here to edit this entry!
