The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) family genes code for key metalloproteinases in the remodeling process of different ECM. Several genes of this family encode for proteins with important functions in reproductive processes; in particular, ADAMTS1, 4, 5 and 9 are genes that are differentially expressed in cell types and the physiological stages of reproductive tissues. ADAMTS enzymes degrade proteoglycans in the ECM of the follicles so that the oocytes can be released and regulate follicle development during folliculogenesis, favoring the action of essential growth factors, such as FGF-2, FGF-7 and GDF-9. The transcriptional regulation of ADAMTS1 and 9 in preovulatory follicles occurs because of the gonadotropin surge in preovulatory follicles, via the progesterone/progesterone receptor complex. In addition, in the case of ADAMTS1, pathways involving protein kinase A (PKA), extracellular signal regulated protein kinase (ERK1/2) and the epidermal growth factor receptor (EGFR) might contribute to ECM regulation. Different Omic studies indicate the importance of genes of the ADAMTS family from a reproductive aspect. ADAMTS genes could serve as biomarkers for genetic improvement and contribute to enhance fertility and animal reproduction.
- animal reproduction
- ADAMTS
- extracellular matrix
- genetic improvement
1. Introduction
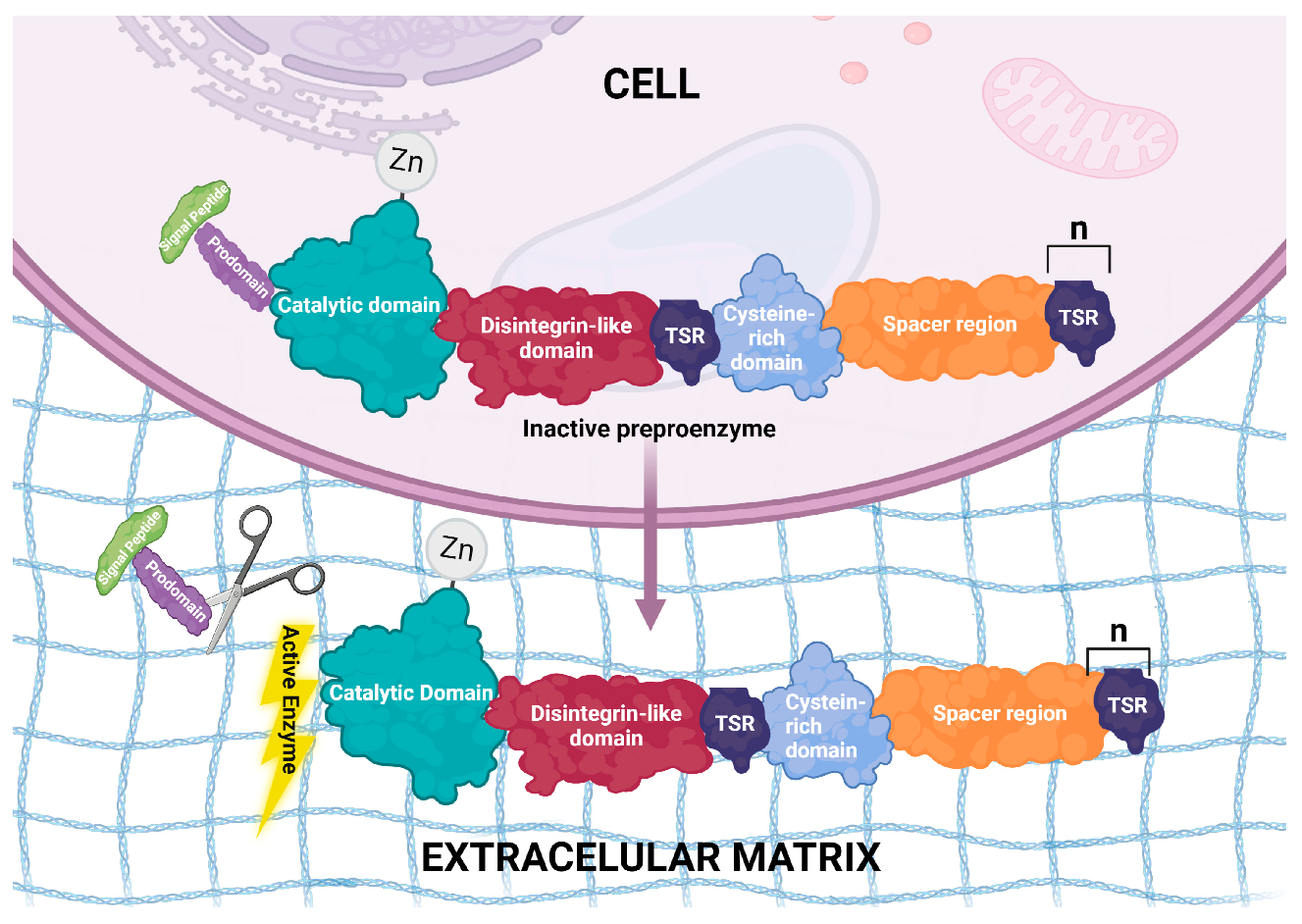
2. Structural Features of ADAMTS Genes
3. ADAMTS and Fertility in Females
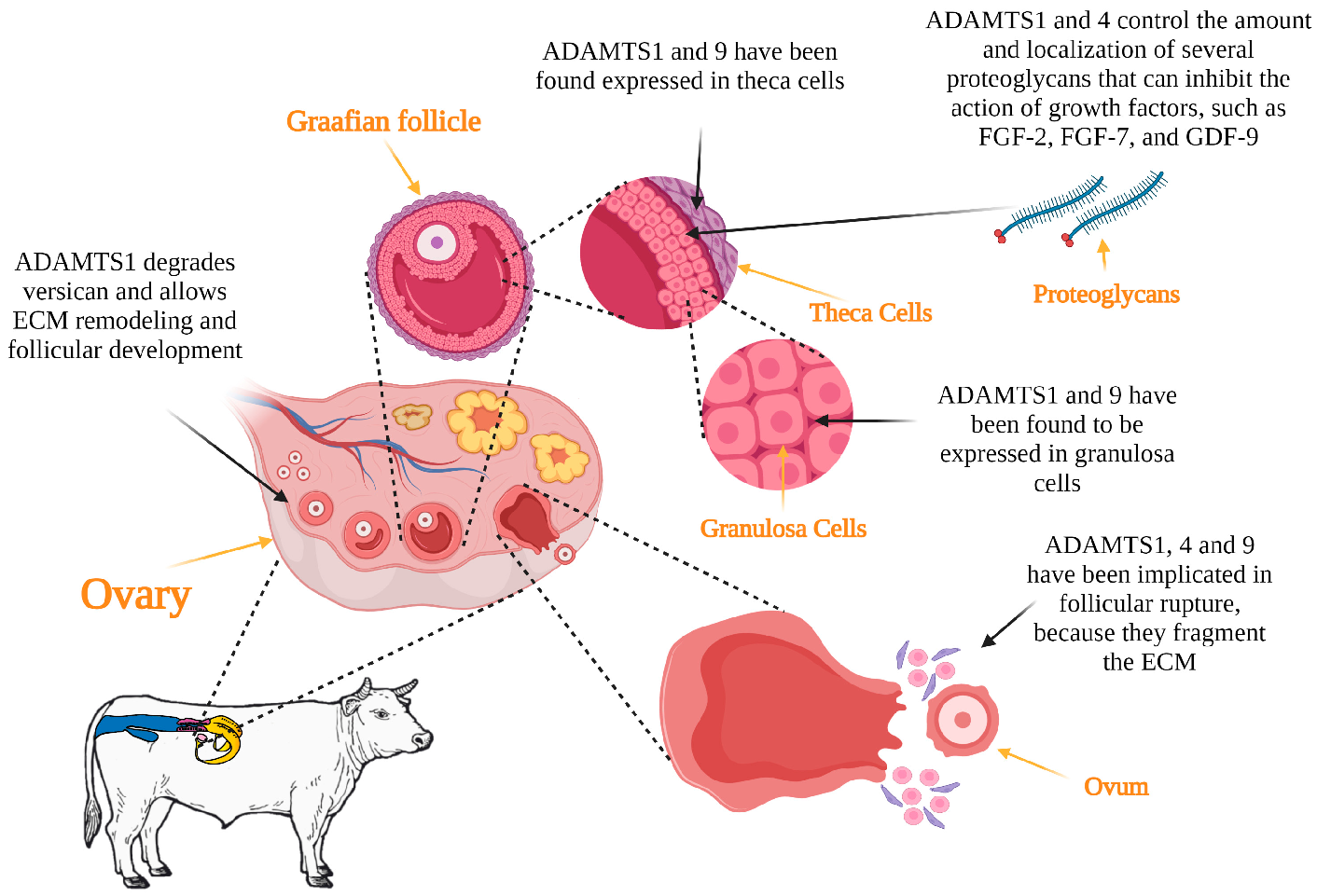
3.1. Folliculogenesis
3.2. Ovulation
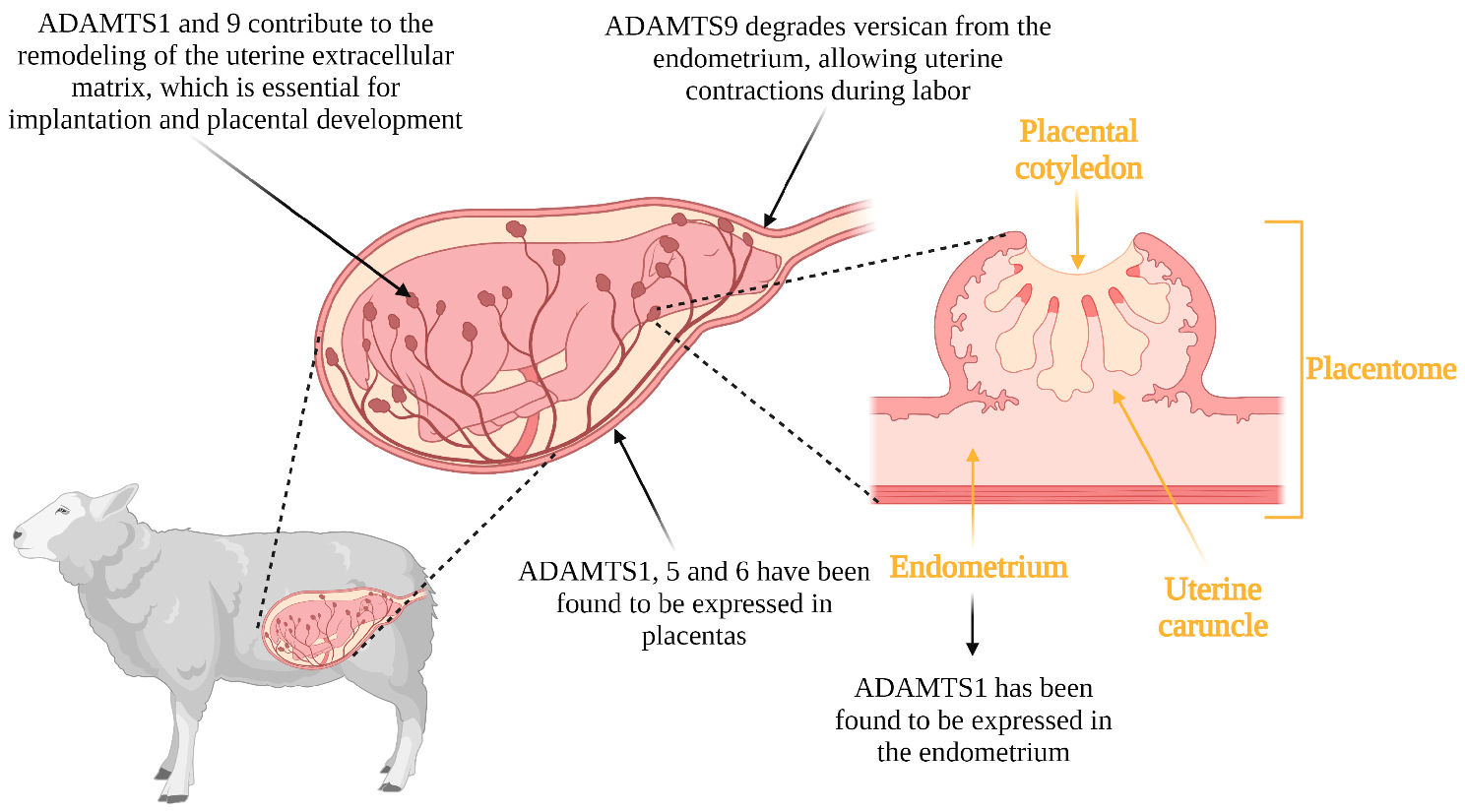
3.3. Implantation, Placentation and Parturition
4. ADAMTS and Fertility in Males
4.1. Testicular Development
4.2. Spermatogenesis
4.3. Fertilization
This entry is adapted from the peer-reviewed paper 10.3390/genes14061181
References
- Hill, W.G. Is continued genetic improvement of livestock sustainable? Genetics 2016, 202, 877–881.
- Taylor, J.F.; Schnabel, R.D.; Sutovsky, P. Genomics of bull fertility. Animal 2018, 12, 172–183.
- Notter, D. Genetic aspects of reproduction in sheep. Reprod. Domest. Anim. 2008, 43, 122–128.
- Albert, F.W.; Kruglyak, L. The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 2015, 16, 197–212.
- Demircan, K.; Comertoglu, I.; Akyol, S.; Yigitoglu, B.N.; Sarikaya, E. A new biological marker candidate in female reproductive system diseases: Matrix metalloproteinase with thrombospondin motifs (ADAMTS). J. Turk. Ger. Gynecol. Assoc. 2014, 15, 250–255.
- Russell, D.L.; Brown, H.M.; Dunning, K.R. ADAMTS proteases in fertility. Matrix Biol. 2015, 44–46, 54–63.
- Copp, H.L.; Shortliffe, L.D. Undescended testes and testicular tumors. In Ashcraft’s Pediatric Surgery; Elsevier: Amsterdam, The Netherlands, 2010; pp. 676–686.
- Dun, M.D.; Anderson, A.L.; Bromfield, E.G.; Asquith, K.L.; Emmett, B.; McLaughlin, E.A.; Aitken, R.J.; Nixon, B. Investigation of the expression and functional significance of the novel mouse sperm protein, a disintegrin and metalloprotease with thrombospondin type 1 motifs number 10 (ADAMTS10). Int. J. Androl. 2012, 35, 572–589.
- Fata, J.; Ho, A.-V.; Leco, K.; Moorehead, R.; Khokha, R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: Functions of metalloproteinases and their inhibitors. CMLS 2000, 57, 77–95.
- Blobel, C.P.; Apte, S. ADAMs and ADAMTSs. In Encyclopedia of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 568–573.
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516.
- Apte, S.S.; Parks, W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015, 44–46, 1–6.
- Dubail, J.; Apte, S.S. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 2015, 44, 24–37.
- Menke, D.B.; Koubova, J.; Page, D.C. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. J. Dev. Biol. 2003, 262, 303–312.
- Doyle, K.M.; Russell, D.L.; Sriraman, V.; Richards, J.S. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol. Endocrinol. 2004, 18, 2463–2478.
- Richards, J.S.; Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Teuling, E.; Lo, Y.; Boerboom, D.; Falender, A.E.; Doyle, K.H.; LeBaron, R.G.; Thompson, V. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: Evidence for specific and redundant patterns during ovulation. Biol. Reprod. 2005, 72, 1241–1255.
- Hurskainen, T.L.; Hirohata, S.; Seldin, M.F.; Apte, S.S. ADAM-TS5, ADAM-TS6, and ADAM-TS7, Novel Members of a New Family of Zinc Metalloproteases. J. Biol. Chem. 1999, 274, 25555–25563.
- Kuno, K.; Kanada, N.; Nakashima, E.; Fujiki, F.; Ichimura, F.; Matsushima, K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J. Biol. Chem. 1997, 272, 556–562.
- Porter, S.; Clark, I.M.; Kevorkian, L.; Edwards, D.R. The ADAMTS metalloproteinases. Biochem. J. 2005, 386, 15–27.
- Apte, S.S. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: Functions and mechanisms. J. Biol. Chem. 2009, 284, 31493–31497.
- Rose, K.W.; Taye, N.; Karoulias, S.Z.; Hubmacher, D. Regulation of ADAMTS proteases. Front. Mol. Biosci. 2021, 8, 701959.
- Huxley-Jones, J.; Apte, S.S.; Robertson, D.L.; Boot-Handford, R.P. The characterisation of six ADAMTS proteases in the basal chordate Ciona intestinalis provides new insights into the vertebrate ADAMTS family. Int. J. Biochem. Cell Biol. 2005, 37, 1838–1845.
- Longpré, J.-M.; Leduc, R. Identification of prodomain determinants involved in ADAMTS-1 biosynthesis. J. Biol. Chem. 2004, 279, 33237–33245.
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113.
- Gershon, E.; Dekel, N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int. J. Mol. Sci. 2020, 21, 4565.
- Madan, P.; Bridges, P.J.; Komar, C.M.; Beristain, A.G.; Rajamahendran, R.; Fortune, J.E.; MacCalman, C.D. Expression of messenger RNA for ADAMTS subtypes changes in the periovulatory follicle after the gonadotropin surge and during luteal development and regression in cattle. Biol. Reprod. 2003, 69, 1506–1514.
- Boerboom, D.; Russell, D.L.; Richards, J.S.; Sirois, J. Regulation of transcripts encoding ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin-like motifs-1) and progesterone receptor by human chorionic gonadotropin in equine preovulatory follicles. J. Mol. Endocrinol. 2003, 31, 473–485.
- Shimada, M.; Nishibori, M.; Yamashita, Y.; Ito, J.; Mori, T.; Richards, J.S. Down-regulated expression of A disintegrin and metalloproteinase with thrombospondin-like repeats-1 by progesterone receptor antagonist is associated with impaired expansion of porcine cumulus-oocyte complexes. Endocrinology 2004, 145, 4603–4614.
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Pritchard, M.; Russell, D.L. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev. Biol. 2006, 300, 699–709.
- Park, P.W.; Reizes, O.; Bernfield, M. Cell surface heparan sulfate proteoglycans: Selective regulators of ligand-receptor encounters. J. Biol. Chem. 2000, 275, 29923–29926.
- Berisha, B.; Sinowatz, F.; Schams, D. Expression and localization of fibroblast growth factor (FGF) family members during the final growth of bovine ovarian follicles. Mol. Reprod. Dev. 2004, 67, 162–171.
- Mishra, S.R.; Thakur, N.; Somal, A.; Parmar, M.S.; Reshma, R.; Rajesh, G.; Yadav, V.P.; Bharti, M.K.; Bharati, J.; Paul, A.; et al. Expression and localization of fibroblast growth factor (FGF) family in buffalo ovarian follicle during different stages of development and modulatory role of FGF2 on steroidogenesis and survival of cultured buffalo granulosa cells. Res. Vet. Sci. 2016, 108, 98–111.
- Matos, M.H.; Lima-Verde, I.B.; Bruno, J.B.; Lopes, C.A.; Martins, F.S.; Santos, K.D.; Rocha, R.M.; Silva, J.R.; Bao, S.N.; Figueiredo, J.R. Follicle stimulating hormone and fibroblast growth factor-2 interact and promote goat primordial follicle development in vitro. Reprod. Fertil. Dev. 2007, 19, 677–684.
- Santos, J.M.; Menezes, V.G.; Barberino, R.S.; Macedo, T.J.; Lins, T.L.; Gouveia, B.B.; Barros, V.R.; Santos, L.P.; Goncalves, R.J.; Matos, M.H. Immunohistochemical localization of fibroblast growth factor-2 in the sheep ovary and its effects on pre-antral follicle apoptosis and development in vitro. Reprod. Domest. Anim. 2014, 49, 522–528.
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Espey, L.L. Ovulation: New dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 2002, 64, 69–92.
- Shozu, M.; Minami, N.; Yokoyama, H.; Inoue, M.; Kurihara, H.; Matsushima, K.; Kuno, K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J. Mol. Endocrinol. 2005, 35, 343–355.
- Russell, D.L.; Ochsner, S.A.; Hsieh, M.; Mulders, S.; Richards, J.S. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology 2003, 144, 1020–1031.
- McArthur, M.E.; Irving-Rodgers, H.F.; Byers, S.; Rodgers, R.J. Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol. Reprod. 2000, 63, 913–924.
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029.
- Richards, J.S.; Russell, D.L.; Robker, R.L.; Dajee, M.; Alliston, T.N. Molecular mechanisms of ovulation and luteinization. Mol. Cell. Endocrinol. 1998, 145, 47–54.
- Russell, D.L.; Doyle, K.M.; Ochsner, S.A.; Sandy, J.D.; Richards, J.S. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 2003, 278, 42330–42339.
- Curry, T.E.; Smith, M.F. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin. Reprod. Med. 2006, 24, 228–241.
- Mittaz, L.; Russell, D.; Wilson, T.; Brasted, M.; Tkalcevic, J.; Salamonsen, L.A.; Hertzog, P.J.; Pritchard, M.A. Adamts-1 is essential for the development and function of the urogenital system. Biol. Reprod. 2004, 70, 1096–1105.
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Boerboom, D.; Pritchard, M.; Lane, M.; Russell, D.L. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 2010, 83, 549–557.
- Hu, W.; Tang, J.; Zhang, Z.; Tang, Q.; Yan, Y.; Wang, P.; Wang, X.; Liu, Q.; Guo, X.; Jin, M.; et al. Polymorphisms in the ASMT and ADAMTS1 gene may increase litter size in goats. Vet. Med. Sci. 2020, 6, 775–787.
- Guillomot, M. Changes in extracellular matrix components and cytokeratins in the endometrium during goat implantation. Placenta 1999, 20, 339–345.
- Nardo, L.G.; Li, T.C.; Edwards, R.G. Introduction: Human embryo implantation failure and recurrent miscarriage: Basic science and clinical practice. Reprod. Biomed. Online 2006, 13, 11–12.
- Guzeloglu-Kayisli, O.; Basar, M.; Arici, A. Basic aspects of implantation. Reprod. Biomed. Online 2007, 15, 728–739.
- Das, S.K.; Yano, S.; Wang, J.; Edwards, D.R.; Nagase, H.; Dey, S.K. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev. Genet. 1997, 21, 44–54.
- Smith, S.E.; Cullen, W.C.; Godkin, J.D. Ultrastructural morphometric analysis of the uterine epithelium during early pregnancy in the sheep. J. Reprod. Fertil. 1990, 89, 517–525.
- Reynolds, L.P.; Redmer, D.A. Growth and microvascular development of the uterus during early pregnancy in ewes. Biol. Reprod. 1992, 47, 698–708.
- Riemer, R.K.; Heymann, M.A. Regulation of uterine smooth muscle function during gestation. Pediatr. Res. 1998, 44, 615–627.
- Mead, T.J.; Du, Y.; Nelson, C.M.; Gueye, N.A.; Drazba, J.; Dancevic, C.M.; Vankemmelbeke, M.; Buttle, D.J.; Apte, S.S. ADAMTS9-Regulated Pericellular Matrix Dynamics Governs Focal Adhesion-Dependent Smooth Muscle Differentiation. Cell Rep. 2018, 23, 485–498.
- Abdul-Majeed, S.; Mell, B.; Nauli, S.M.; Joe, B. Cryptorchidism and infertility in rats with targeted disruption of the Adamts16 locus. PLoS ONE 2014, 9, e100967.
- Jacobi, C.L.; Rudigier, L.J.; Scholz, H.; Kirschner, K.M. Transcriptional regulation by the Wilms tumor protein, Wt1, suggests a role of the metalloproteinase Adamts16 in murine genitourinary development. J. Biol. Chem. 2013, 288, 18811–18824.
- Sarila, G.; Bao, T.; Abeydeera, S.A.; Li, R.; Mell, B.; Joe, B.; Catubig, A.; Hutson, J. Interplay between collagenase and undescended testes in Adamts16 knockout rats. J. Pediatr. Surg. 2020, 55, 1952–1958.
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, 27–35.
- Gurupriya, V.S.; Roy, S.C.; Javvaji, P.K.; Dhali, A.; Badami, S.; Rahim, F.; Divyashree, B.C.; Panda, A.P. Expression of ADAMTS10 in male reproductive tract of buffaloes (Bubalus bubalis). J. Exp. Biol. Agric. Sci. 2018, 6, 800–807.
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227.
- Li, S.W.; Arita, M.; Fertala, A.; Bao, Y.; Kopen, G.C.; Langsjo, T.K.; Hyttinen, M.M.; Helminen, H.J.; Prockop, D.J. Transgenic mice with inactive alleles for procollagen N-proteinase (ADAMTS-2) develop fragile skin and male sterility. Biochem. J. 2001, 355, 271–278.
- González-Barrio, D.; Diezma-Díaz, C.; Gutiérrez-Expósito, D.; Tabanera, E.; Jiménez-Meléndez, A.; Pizarro, M.; González-Huecas, M.; Ferre, I.; Ortega-Mora, L.M.; Álvarez-García, G. Identification of molecular biomarkers associated with disease progression in the testis of bulls infected with Besnoitia besnoiti. Vet. Res. 2021, 52, 106.
- Olias, P.; Schade, B.; Mehlhorn, H. Molecular pathology, taxonomy and epidemiology of Besnoitia species (Protozoa: Sarcocystidae). Infect. Genet. Evol. 2011, 11, 1564–1576.
- Wu, S.; Mipam, T.; Xu, C.; Zhao, W.; Shah, M.A.; Yi, C.; Luo, H.; Cai, X.; Zhong, J. Testis transcriptome profiling identified genes involved in spermatogenic arrest of cattleyak. PLoS ONE 2020, 15, e0229503.
- Le Goff, C.; Cormier-Daire, V. The ADAMTS(L) family and human genetic disorders. Hum. Mol. Genet. 2011, 20, R163–R167.
