Thermoelectric materials have gained wide attention to realize multilevel efficient energy management to alleviate the increasingly severe energy crisis. Oxide ceramics were well-explored as potential thermoelectric candidates because of their outstanding merits, including abundance, eco-friendliness, high-temperature stability, and chemical stability. In this work, we aim to provide a comprehensive summary of the diversified state-of-the-art oxide ceramics and establish the links between composition designing, preparation process, structural characteristics, and properties to summarize the underlying chemistry and physics mechanism of band engineering, doping, composited with the second phase, defects engineering, and entropy engineering. Furthermore, advanced device design and applications such as thermoelectric modules, miniature generators, sensors, and coolers were reviewed. Ultimately, the challenges and future perspective of oxides ceramics for the device design and thermoelectric applications in the development of energy harvesting technology have been prospected.
- thermoelectrics
- oxides ceramics
- ZT
- electrical conductivity
- phonon scattering
1. Introduction
Thermoelectric materials (TEs) have been used as a potential energy harvesting technology because they can convert heat into electricity and have no requirements for waste heat temperature [1,2,3]. Thermoelectric devices generally consist of n-type and p-type TEs wired electrically in series (or partly parallel) and thermally in parallel. Furthermore, there are also thermoelectric devices with single n-type and p-type TEs. They have the advantages of no moving parts, no noise, small size, etc., and have significant application merits in the military, aerospace, and high-tech energy fields [4,5].
It has been more than 200 years since the thermoelectric effect was discovered, and people have been constantly exploring and developing its industrial applications. In the early 1920s, Altenkirch, a German physicist, developed the fundamentals of thermoelectric power generation and refrigeration and summarized the performance evaluation parameters of TEs [6]: electrical conductivity (σ), Seebeck coefficient (S), and thermal conductivity (κ). Dimensionless thermoelectric merit (ZT = S2σT/κ, S is Seebeck coefficient, σ is electrical conductivity, T is absolute temperature, κ is thermal conductivity) is usually used as an indicator to measure the thermoelectric performance [7]. TEs with large ZT values must meet the requirements of a high Seebeck coefficient to ensure the generation of the obvious thermoelectric effect—high electrical conductivity leading to small Joule heat, large output power, as well as low thermal conductivity, are required to generate a substantial temperature difference. The above three thermoelectric parameters have a strong coupled relationship because they are dependent on the carrier concentration in a conflicting manner that restricts and influences each other, making how to optimize thermoelectric performance a huge challenge. Therefore, the coordinated regulation of S, σ, and κ to improve ZT has become the key point to realize the industrial application of thermoelectric materials.
2. Thermoelectric Fundamentals
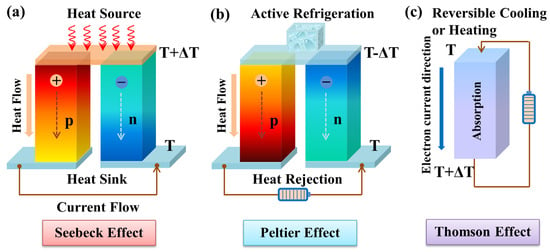
Figure 2. Schematic diagram of thermoelectric effects. (a) Seebeck effect for power generation, (b) Peltier effect for refrigeration, (c) Thomson effect for reversible cooling or heating.
3. Fabrication of Thermoelectric Oxide Ceramics
3.1. Lattice Structures of Thermoelectric Oxide Ceramics
3.1.1. n-Type Thermoelectric Oxides
3.1.2. p-Type Thermoelectric Oxides
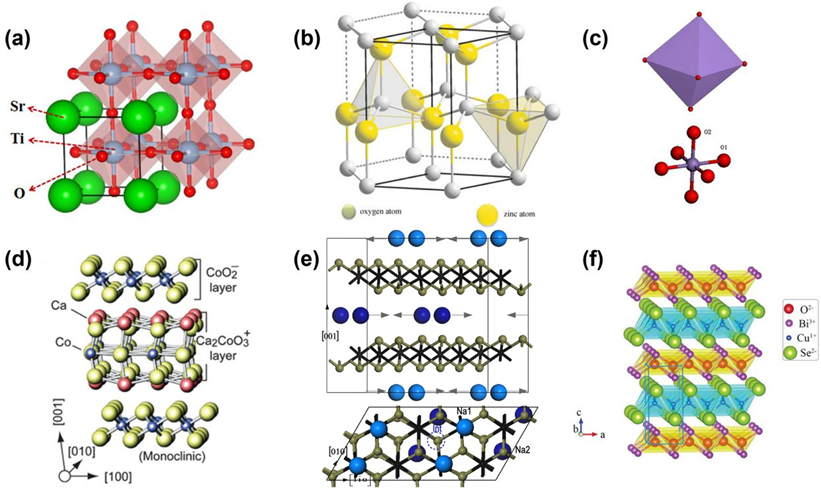
Figure 3. Schematic of the crystal structure of thermoelectric oxides. (a) SrTiO3, (b) ZnO [43], (c) CaMnO3 [29], (d) Ca3Co4O9 [9], (e) NaxCoO2 [16], (f) BiCuSeO [21].
3.2. Preparation Method
3.2.1. Bulk Crystal Growth
3.2.2. Solid-State Method
3.2.3. Spark Plasma Sintering
3.2.4. Mechanical Alloying
3.2.5. Liquid-Phase Synthesis
Colloidal Synthesis
4. Development and Strategies to Improve the Thermoelectric Performance of Oxide Ceramics
Several of the selection rules are somewhat paradoxical as a result of the inherent trade-off effect between σ and S. Many optimized strategies may have a complicated correlated impact on S, σ, and κ. As an illustration, the increase of doping concentration will improve σ while decreasing S. Then, Ioffe’s finding in doped semiconductors was the first effort to empirically determine the carrier concentration “sweet spot” of excellent thermoelectrics is n = 1018–1020 cm−3 [86]. Consequently, an optimal power factor (PF = S2σ) versus doping concentration exists at a relatively high doping level. Furthermore, a further decrease of κ is necessary to produce a high ZT.
4.1. Band Engineering
4.2. Doping
4.3. Entropy Engineering
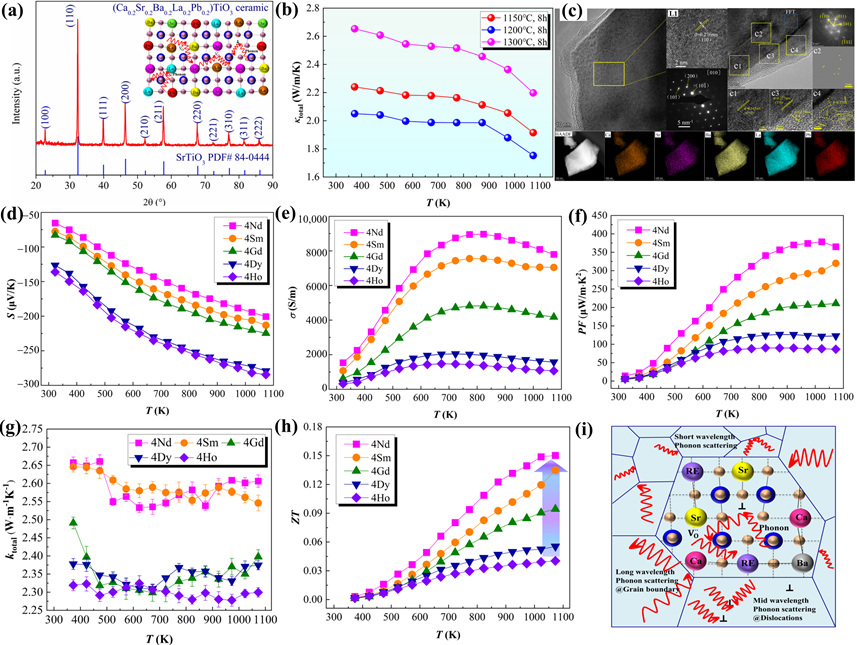
Figure 7. (a) XRD patterns, (b) κ, (c) TEM results of the high-entropy (Ca0.2Sr0.2Ba0.2La0.2Pb0.2)TiO3 ceramics [59]. (d–h) S, σ, PF, κtotal, and ZT values of the high-entropy (Sr0.2Ca0.255Ba0.25RE0.25)TiO3 ceramics [134]. (i) Schematic diagram of the possible phonon scattering in reducing lattice thermal conductivity for high-entropy ceramic [135].
4.4. Defect Engineering
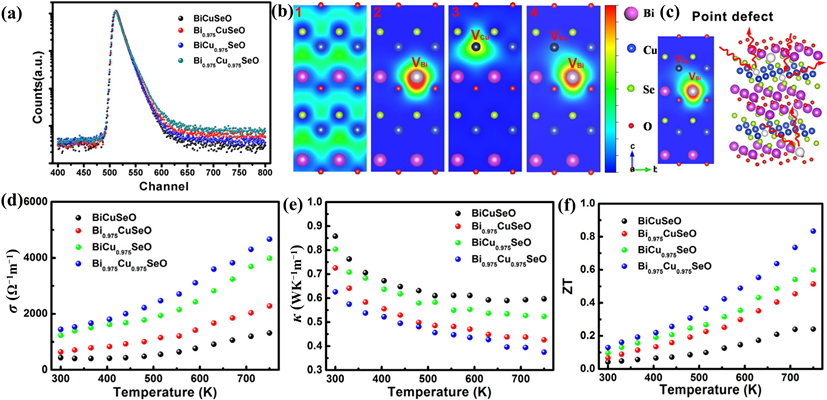
Figure 8. (a) Positron lifetime spectrum. (b) Schematic representation of trapped positrons for Bi1–xCu1–ySeO samples in (100) plane. (c) Schematic representation of phonon scattering with Bi/Cu vacancies. (d–f) σ, κ, and ZT values of the Bi1–xCu1–ySeO samples [148].
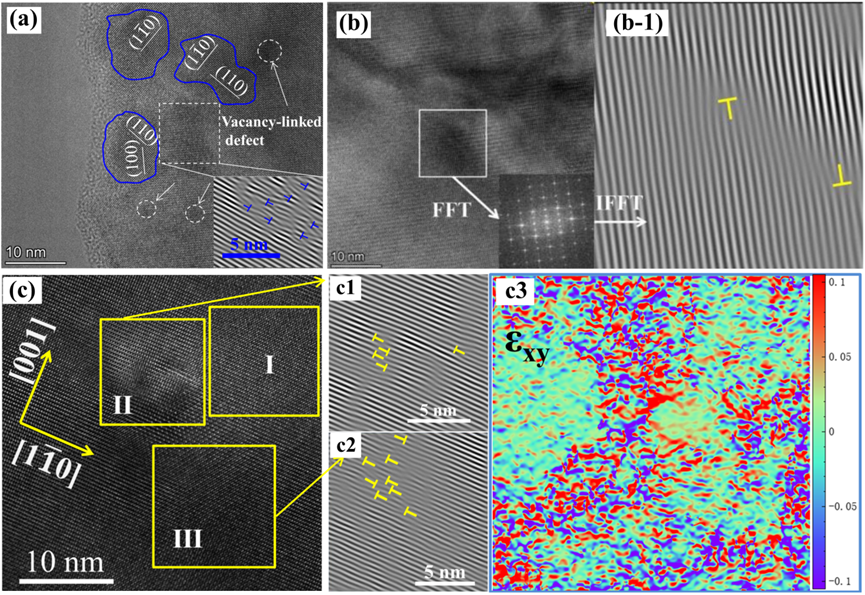
4.5. Grain Boundary and Nanostructure Engineering
4.6. Textured Engineering
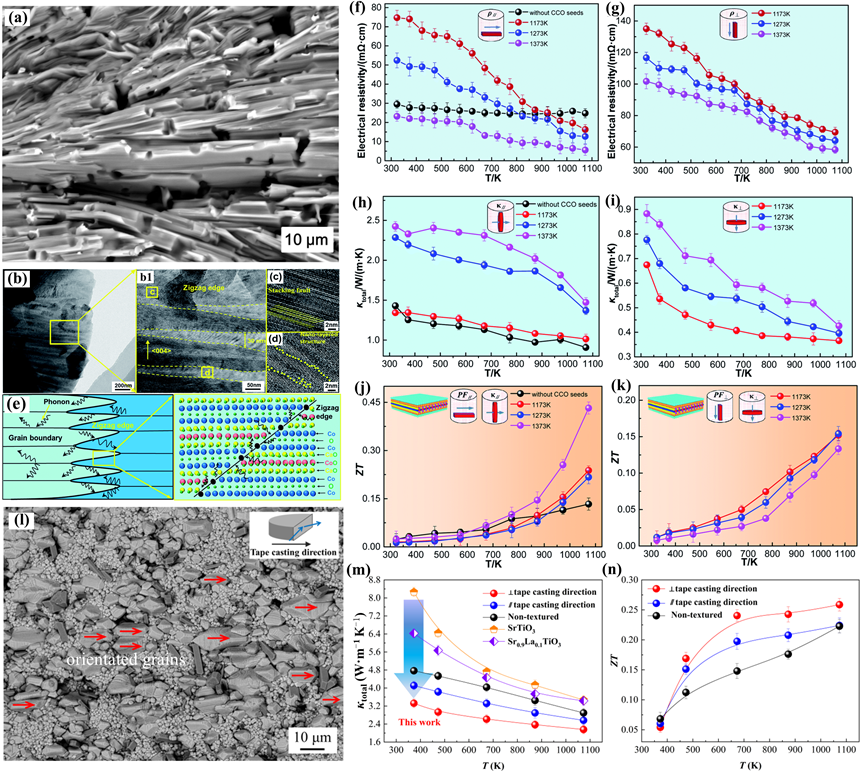
Figure 11. (a) BSE image, (b–d) TEM images, and (e) the sketch diagram of phonon scattering at the grain boundary and zigzag edge of the (Ca0.87Ag0.1La0.03)3Co4O9 textured ceramics, and temperature dependence of the thermoelectric properties of the samples in parallel and perpendicular to the tape-casting direction. (f,g) Electrical resistivity, (h,i) thermal conductivity, (j,k) ZT values [12]. (l) BSE image, (m) Total thermal conductivity, and (n) ZT values of the Sr0.9La0.1TiO3-based textured ceramic [156].
4.7. Composites
5. Device Applications
5.1. Power Generation
5.2. Sensor Devices
5.3. Flexibility and Wearable Devices
5.4. Other Application
This entry is adapted from the peer-reviewed paper 10.3390/en16114475
