Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Alkaloids are organic chemical compounds with a cyclic ring structure containing one or more basic nitrogen atoms. They are widely distributed in nature and are found as naturally occurring secondary metabolites in both plants and animals.
- alkaloids
- antineoplastic
- plants
1. Introduction
Cancer is the common name given to a group of complex pathologies triggered by factors that damage genetic materials, thus resulting in uncontrolled cell proliferation and consequent formation of abnormal cells capable of attacking other near or distant cells [1]. While genetic alterations in normal tissues can also result in benign and premalignant tumors, there are indications that major health challenges associated with cancer diseases, especially those without an early diagnosis, are significantly linked to cancerous or malignant tumors [2]. According to Sung et al. [3], cancer is the principal cause of human mortality and a limitation to long life expectancy in many developed and developing countries. With about 19.3 million people affected in 2020, deaths due to this disease amounted to 10 million out of the former estimate. Moreover, by 2040, the worldwide health challenge due to cancer is projected to increase by 47%. Cancer has the possibility of developing in nearly any tissue or organ in the body. However, as of 2020, breast (2.26 million), lung (2.21 million), prostrate (1.41 million), skin (1.19 million) and colon (1.14 million) cancers represented the leading types of this pathology documented globally [3].
Currently, various clinically approved treatment modalities exist for cancer treatment. These include chemotherapy, radiotherapy, immunotherapy, hormone therapy and targeted therapy, which may be applied alone as monotherapy or together as a combined therapy. However, despite the success recorded with these therapeutic regimes, their application in cancer management is challenged by unwanted adverse effects, such as lack of specificity or toxicity with regard to normal cells, disease re-emergence years after treatment and poor bioavailability, amongst others [4][5]. Consequently, these factors, combined with the high cost of conventional cancer therapies, have led many people, especially those in developing countries, to explore pharmacologically active chemical compounds present in plant products as alternative treatment options [6].
Plants have been described as reservoirs of bioactive compounds for cancer treatment [7]. Newman and Cragg [8] revealed that about 35% of all anticancer drugs available for cancer management between 1981 and 2014 were obtained from plant products. Alkaloids represent one of the highly diverse categories of chemical compounds in plants that regulate plant growth and protect them against herbivory [9]. They have structural diversity, which results in them possessing various pharmacological properties, including anticancer effects. While alkaloids such as vinblastine first identified from plants are now clinically approved for cancer treatment, poor solubility, low bioavailability, drug resistance and hepatic toxicity have limited experiments with some alkaloids from advancing beyond in vitro and in vivo experiments.
2. Alkaloids and Their General Applications
Alkaloids are organic chemical compounds with a cyclic ring structure containing one or more basic nitrogen atoms. They are widely distributed in nature and are found as naturally occurring secondary metabolites in both plants and animals. Despite this distribution, screening, identification and discovery of pharmacologically relevant alkaloids with usefulness in disease management have been carried out mainly with those chemical candidates derived from plant sources [10]. While they are primarily synthesized from amino acids, alkaloids can be found in seeds, roots, stems and leaves of higher plants, such as those in the Solanaceae, Ranunculaceae, Loganiaceae, Menispermaceae, Amaryllidaceae and Papaveraceae families [11]. Although alkaloids’ function in plants is complex and not fully understood, there are indications that their production in plants could be attributed to defensive evolution against biotic factors, such as pathogens, insects and animals, that could pose threats to their existence as hosts. Chen et al. [12] showed that 7-demethoxytylophorine, a phenanthroindolizidine alkaloid, has antifungal properties, with a minimum inhibitory concentration of 1.56 µg mL−1 against Penicillium italicum, which is responsible for blue mold disease in plants [13]. Moreover, Hikal et al. [14] reviewed the insecticidal activities of quinolone, pyridine and piperidine alkaloids against pests affecting various different plants.
According to Heinrich et al. (2021), alkaloids possess some unique chemical characteristics that make them interesting candidates for use in medicine. In their basic state, they are soluble under acidic conditions, whereas they become lipid membrane-permeable when neutral after losing their protons. With these properties, alkaloids have been used in various applications in plant and human disease treatments (Figure 1). Currently, morphine and its chemical derivative codeine, obtained from Papaver somniferu, are often used as analgesics. Before replacement with other effective plant-derived drugs (e.g., artemisine), the antimalarial properties of quinine were well explored following the 17th century [15]. Tubocurarine and ergonovine, from claviceps and ephedra plant species, respectively, have been used to suppress bleeding due their effects in narrowing blood vessels. Alkaloids, including ephedrine and atropine, have been administered to treat respiratory illnesses, while others, such as vincristine, berberine and vinblastine, have been employed as anticancer agents [9].
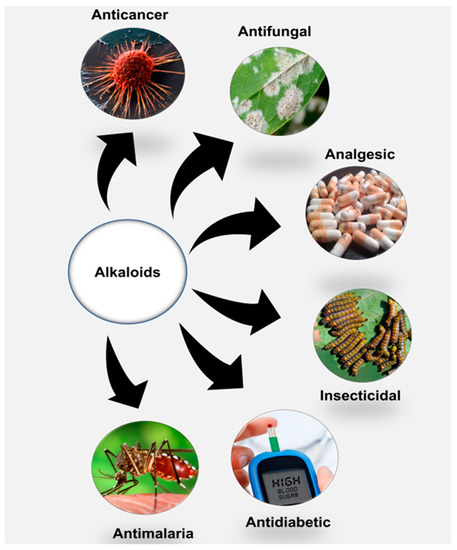
Figure 1. Alkaloids and their general pharmacological significance.
3. Alkaloids in Cancer Treatment: A Historical Perspective
The first type of alkaloid that was applied in cancer therapy was discovered in the 1950s accidentally from Vinca rosea, a plant that is also referred to as Catharanthus roseus, by two researchers; namely, Charles Beer and Robert Noble [16]. Knowledge of the traditional use of this plant in reducing blood glucose led them to investigate its hypoglycemic effect in rats. However, instead of these antihyperglycemic properties, they discovered a considerable reduction in bone marrow damage in these animals with a simultaneous decrease in blood leucocytes and granulocytes [17]. After this finding, more focused studies on rats with lymphocytic leukemia led to the characterization of vinblastine as the first clinical anticancer use of Vinca alkaloids. To date, many other chemical compounds of the same plant category, including colchicine, vinflunine, vincamine, vinorelbine and vincristine, have also been documented for their antitumor efficacies [18][19].
4. Chemistry of Anticancer Alkaloids
Alkaloids are a very diverse group of plant secondary metabolites. There is no uniform pattern of classifying them due to their structural variation, as observed in Figure 2, which often does not correlate with their biological activities. For instance, alkaloids with similar ring structures but synthesized from different metabolic pathways could possess different pharmacological activities [20]. Consequently, alkaloids can be classified depending on the type of plant families they are derived from. Typical examples of this taxonomical classification include vinblastine, present in Vinca rosea, an alkaloid from the plant family Apocyanaeae; lobeline, found in Lobelia inflata as derived from the Lobeliaceae family; and physostigmine, present in Physostigma venenosum as derived from the family Leguminosae [21].
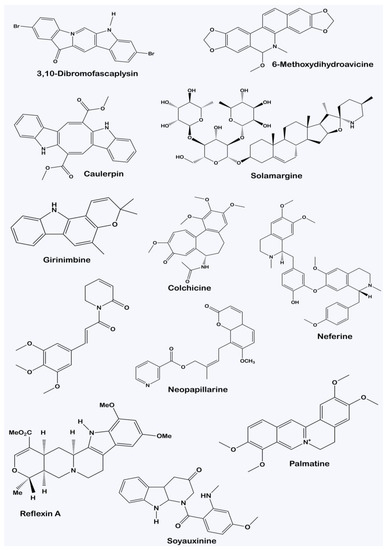
Figure 2. Structural diversity of alkaloids characterized from various plants.
Another form of alkaloid classification is based on the precursor biomolecule used as the starting material for their biosynthetic pathways. Under this classification, alkaloids produced from the same amino acids, like arginine, tyrosine, proline, tryptophan, lysine and aspartate, can all be grouped in a class despite possessing different pharmacological activities and taxonomic distributions [22]. However, according to Dey et al. [20], the best all-inclusive classification of these basic nitrogen-containing phytochemicals involves categorizing them based on their chemical structures into tropane, pyrrolizidine, piperidine, quinoline, isoquinoline, indole, steroidal, imidazole, purine and pyrrolidine alkaloids.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28145578
References
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868.
- Patel, A. Benign vs Malignant Tumors. JAMA Oncol. 2020, 6, 1488.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Amini, P.; Moazamiyanfar, R.; Dakkali, M.S.; Khani, A.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Johari, R.; Taeb, S.; Najafi, M. Resveratrol in Cancer Therapy: From Stimulation of Genomic Stability to Adjuvant Cancer Therapy: A Comprehensive Review. Curr. Top Med. Chem. 2022, 23, 629–648.
- Eisenmann, E.D.; Talebi, Z.; Sparreboom, A.; Baker, S.D. Boosting the oral bioavailability of anticancer drugs through intentional drug–drug interactions. Basic Clin. Pharmacol. Toxicol. 2022, 130, 23–35.
- Sarbadhikary, P.; George, B.P. A Review on Traditionally Used African Medicinal Plant Annickia chlorantha, Its Phytochemistry, and Anticancer Potential. Plants 2022, 11, 2293.
- Olawale, F.; Iwaloye, O.; Olofinsan, K.; Ogunyemi, O.M.; Gyebi, G.A.; Ibrahim, I.M. Homology modelling, vHTS, pharmacophore, molecular docking and molecular dynamics studies for the identification of natural compound-derived inhibitor of MRP3 in acute leukaemia treatment. Chem. Pap. 2022, 76, 3729–3757.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661.
- Joanna, K. Introductory Chapter. In Alkaloids–Their Importance in Nature and for Human Life; Joanna, K., Ed.; IntechOpen: Rijeka, Croatioa, 2019.
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids used as medicines: Structural phytochemistry meets biodiversity—An update and forward look. Molecules 2021, 26, 1836.
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mat. Today Chem. 2018, 9, 56–72.
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory effect of 7-demethoxytylophorine on Penicillium italicum and its possible mechanism. Microorganisms 2019, 7, 36.
- Kanashiro, A.M.; Akiyama, D.Y.; Kupper, K.C.; Fill, T.P. Penicillium italicum: An underexplored postharvest pathogen. Front. Microbiol. 2020, 11, 606852.
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274.
- Shanks, G.D. Historical review: Problematic malaria prophylaxis with quinine. Am. J. Trop. Med. Hyg. 2016, 95, 269.
- Banyal, A.; Tiwari, S.; Sharma, A.; Chanana, I.; Patel, S.K.S.; Kulshrestha, S.; Kumar, P. Vinca alkaloids as a potential cancer therapeutics: Recent update and future challenges. 3 Biotech 2023, 13, 211.
- Zhang, Y.; Yang, S.H.; Guo, X.L. New insights into Vinca alkaloids resistance mechanism and circumvention in lung cancer. Biomed. Pharmacother. 2017, 96, 659–666.
- Gerullis, H.; Wawroschek, F.; Köhne, C.-H.; Ecke, T.H. Vinflunine in the treatment of advanced urothelial cancer: Clinical evidence and experience. Ther. Adv. Urol. 2017, 9, 28–35.
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206.
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567.
- Singh, R. Chemotaxonomy: A tool for plant classification. J. Med. Plants Stud. 2016, 4, 90–93.
- Eguchi, R.; Ono, N.; Hirai Morita, A.; Katsuragi, T.; Nakamura, S.; Huang, M.; Amin, A.U.M.; Kanaya, S. Classification of alkaloids according to the starting substances of their biosynthetic pathways using graph convolutional neural networks. BMC Bioinform. 2019, 20, 380.
This entry is offline, you can click here to edit this entry!
