Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The secondary metabolome of fungi is vast and largely unexplored, especially from Ascomycetes and Basidiomycetes, which comprise filamentous fungi. They are the source of many compounds with medical, industrial, and agricultural importance.
- filamentous fungi
- fungal secondary metabolites
- bioprocess
1. Fungal Secondary Metabolites
The secondary metabolome of fungi is vast and largely unexplored, especially from Ascomycetes and Basidiomycetes, which comprise filamentous fungi [1]. They are the source of many compounds with medical, industrial, and agricultural importance [2].
Filamentous fungi provide several ecosystem functions including the following: the symbiotic ones have a close, mutually beneficial relationship with a nonfungal organism; the saprobic ones utilize the nutrients released when breaking down debris; and parasitic fungi feed by taking away nutrients from another organism [3]. One strategy employed by several fungi to stay ecologically relevant is to produce antibiosis molecules, and some of them can be used as medicine [4]. The niches where they belong are very competitive, so on top of the pure weaponization of their secondary metabolome, they also produce a multitude of signal molecules that may have other valuable properties. For example, 5-methyl-phenazine-1-carboxylic acid (1) is an antifungal drug, but in the wild, it is used in smaller quantities as a signal to induce asexual sporulation [1][5]. Other fungal metabolites have different but potentially interesting effects, like emodin (2), which only has a moderate antibacterial effect but significantly enhances the efficacy of oxacillin against MRSA when combined with it [6], and avellanin C (3, Figure 1), which has anti-quorum sensing properties in S. aureus [7].
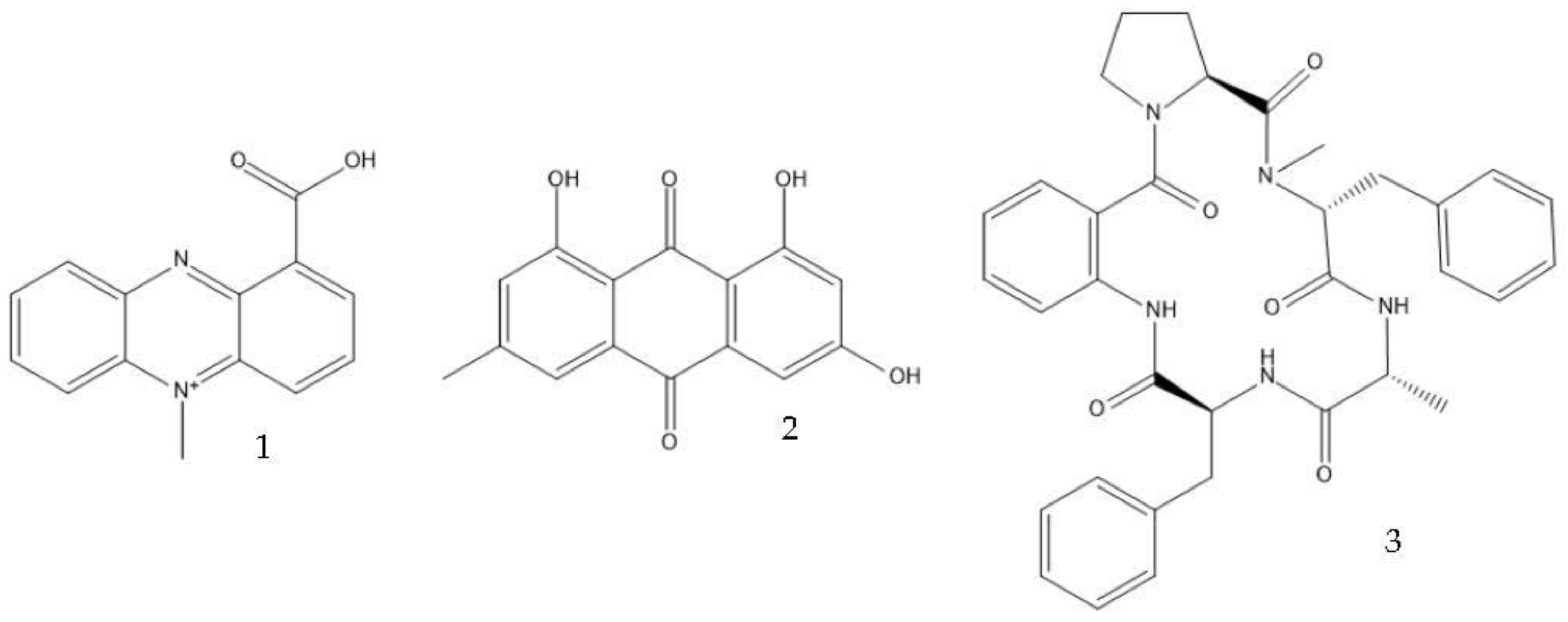
Figure 1. Structures of 5-methyl-phenazine-1-carboxylic acid (1), emodin (2), and avellanin C (3).
The three most important classes of secondary metabolites from fungi are polyketides, derived from acyl CoA precursors; non-ribosomal peptides that condense amino acids in pathways involving enzymes instead of ribosomes; and terpenoids formed by mevalonic-acid-derived units [8][9][10].
Many antibiotics have been discovered from filamentous fungi. Penicillins like penicillin V (4, Figure 2) and cephalosporines like cephalosporine C (5) were the first and second classes that were discovered, respectively. They are chemically very similar, as both are non-ribosomal peptides that have a β-lactam ring (four-atom cyclic amide) that binds to peptidoglycans and prevents them from cross-linking, compromising the stability of the cell walls, especially from Gram-positive bacteria [3][11]. Pleuromutilin (6) and its derivatives have taken a long time to enter the human market despite being discovered in the “golden age” of antibiotics. They block the start of protein synthesis by interfering with the 50S bacterial ribosomal unit [12]. Enniatins like fusafungine (7) are produced by mycotoxin-producing Fusarium spp. They interfere with the cell wall and disrupt the cell membrane, but show other effects such as the inhibition of drug efflux pumps and MDR phenotype expression [13][14]. Fusidic acid (8) is the only member of its class and is a narrow-spectrum antibiotic that is commonly used against S. aureus. It binds to an elongation factor and the corresponding ribosome during protein synthesis, which does not allow it to continue protein synthesis. While eukaryotes have other elongation factors, bacteria become unable to synthesize proteins [15].
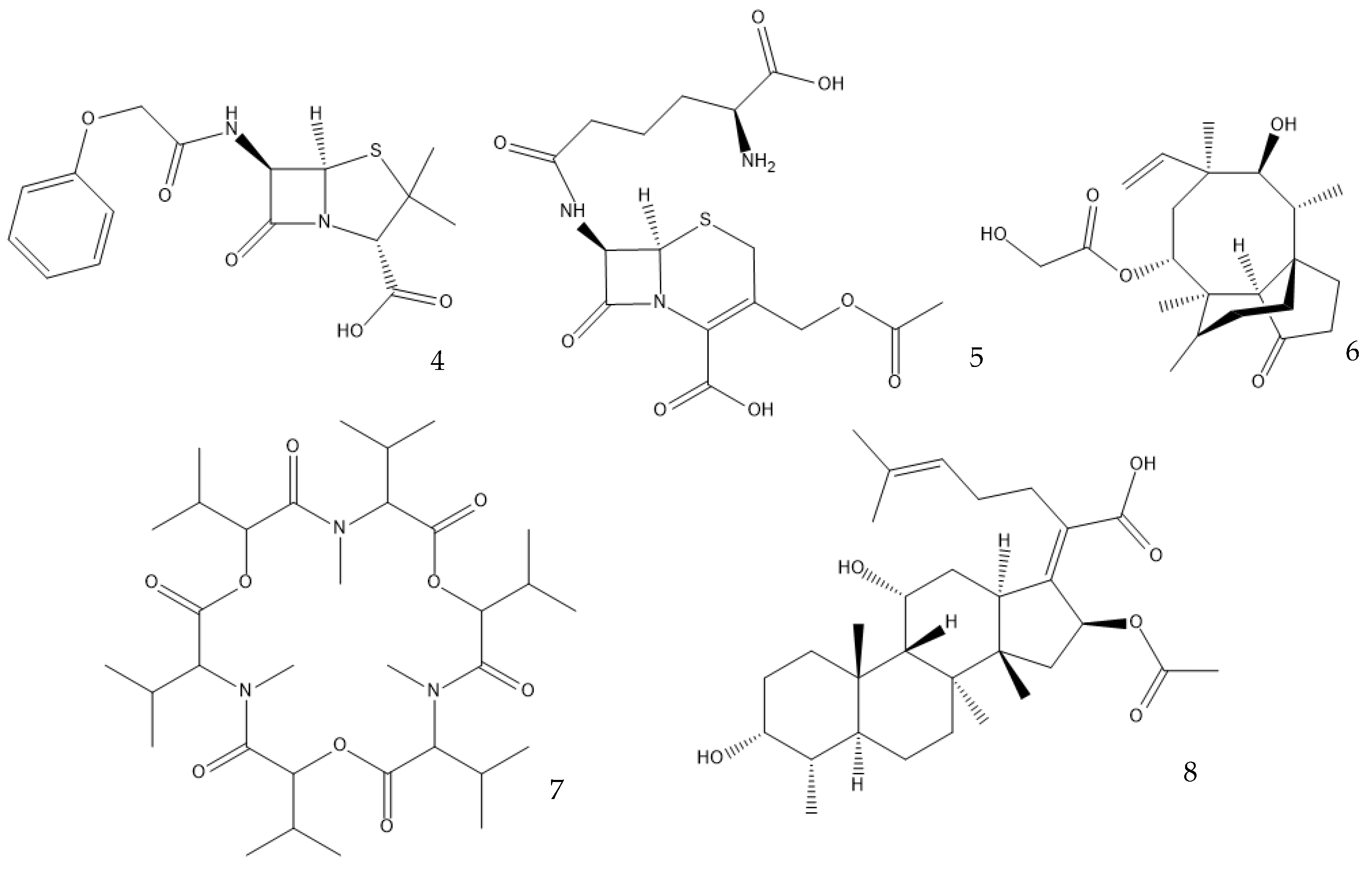
Figure 2. Structures of penicillin V (4), cephalosporine C (5), pleuromutilin (6), fusafungine (7), and fusidic acid (8).
One of the most challenging aspects of discovering new drugs from fungi is their production at a large scale, as standard laboratory conditions are often not suitable for that purpose [16].
2. Approaches to Secondary Metabolite Production from Fungi
2.1. OSMAC Approach
Culture conditions are of utmost importance in determining which compounds will be synthesized and in which quantities. There are countless examples of effects, but a relevant one is an extract of Bulgaria inquinans, which accumulated four new metabolites only when a mixture of inorganic salts was added to the culture [17]. Even the harmless change from tap water to distilled water in the making of the culture media promoted the production of six new secondary metabolites in a Paraphaeosphaeria quadriseptata culture [18]. The “One Strain MAny Compounds” (OSMAC) approach may be incredibly successful at enhancing the chemical diversity obtained, as changing culture conditions like the pH, temperature, aeration, and carbon and nitrogen sources can alter the metabolite profile of the fungi. Potato dextrose or malt extract are both great to maintain fungal cultures but often fail in terms of secondary metabolite production. Adding enzyme inducers/inhibitors or chemical elicitors can also change what is produced [16].
The discovery and commercialization of penicillin had a significant time gap, mostly due to the time it took to develop an effective large-scale production method. The first versions of this method used a broth consisting of lactose (3–4%), corn steep liquor (4%), CaCO3 (1%), KH2PO4 (0.4%), and antifoam (0.25%). Over time, penicillin was improved, but the main difference is the replacement of lactose by glucose or molasses at 10%, which are cheaper. Phenylacetic acid, or another molecule that may serve as a side chain, was also added, as its synthesis is the limiting step. Corn steep liquor is advantageous for its price and chemical diversity, containing large amounts of micronutrients, nitrogen, and side-chain precursors [19][20]. The maximum volume of the reactors used increased, as new technologies allow for better oxygen diffusion, which is essential for the production of most secondary metabolites, including penicillin, but oxygen diffusion is a challenge, as fungus significantly increases the viscosity of the medium [19]. The morphology acquired by the cells influences the production of secondary metabolites, and this aspect needs to be optimized. It was shown that the formation of pellets does not directly affect the ability of Penicillium chrysogenum to produce penicillin, but reduces the medium viscosity, which, in turn, increases the diffusion rate of respiratory gases, reducing energy costs and increasing productivity. The morphology can be influenced by diverse aspects, such as the spore density of the inoculum, the agitation speed, or the CO2 concentration [19][21][22]. The strain that is used also changed significantly, and all factors are important. This increased the concentration of penicillin obtained in the broth from 0.001 g/L in the year 1939 to the current concentrations of over 50 g/L [9][19].
2.2. Liquid- and Solid-State Fermentations
Secondary metabolites can be produced via fermentation in a liquid broth; via solid support, like the Lightweight Expanded Clay Aggregates (LECA) “nuts”; or most often, by the use of a gelling agent like agar media, which consistently gives good results [16].
Submerged fermentation is often preferred in industrial processes because it is easier to scale up, monitor, and automate. A large scale is often essential for industrial-scale viability, and contrary to solid-state fermentation, it avoids the build-up of temperature, pH, and O2 concentration gradients in large reactors [23]. The purification of the products also becomes easier, but it ends up generating a great quantity of wastewater [24]. Liquid fermentation is notably fast, usually lasting only 1–2 weeks, so it requires diverse equipment to manage in real time. Frequently, the constant influx of nutrients supplied by a fed-batch stream is required, as nutrients in a liquid medium are easy to access, and thus are rapidly consumed by microorganisms [19][24]. In mushroom fermentations, using a liquid-state system does not allow the formation of fruiting bodies that sometimes contain bioactive molecules [25].
Submerged fermentation at an industrial scale must carefully consider several physical, chemical, and biological parameters that can severely influence the success of the operation. Among them, agitation and aeration can significantly increase energy consumption. This energy adds to that released from the fungal metabolism, so it requires an effective cooling system to maintain an appropriate temperature for the microorganism [19][26][27]. At a laboratory scale, it is easier to maintain an adequate temperature and aeration, given that the volume of a reactor with its walls and bottom is much smaller than in an industrial fermenter. Additionally, a small pump can provide a relatively large volume of air. However, even these reactors may create concentration gradients and agitation dead zones [27]. These parameters must be carefully considered before scaling-up operations. It is common to express the agitation used in these experimental setups in rotations per minute (rpm), but this fails to precisely express the forces undergone by the broth. The use of the agitation Reynolds number or the mixing time is thus easier to compare between independent experiments, especially at different scales. It is important to consider that the fermentation broth of filamentous fungi is usually a viscous, non-Newtonian fluid, so its viscosity depends on the shear rate, and more heavily so when the mycelium does not grow in pellets [21][27][28]. In an Aspergillus niger culture, it was found that the optimal rotation frequency for pectinase production was 150 rpm. While a slower agitation did not promote a significant mixture, higher speeds increased the shear force experienced by the cells and decreased the enzyme activity, while still increasing the biomass production, up to 200 rpm [29]. Another study on the same species directed for the production of tannase demonstrated that the optimal agitation speed was 130 rpm for enzyme activity and 100 rpm for growth optimization [30].
Solid-state fermentation may be described as occurring in a solid matrix that contains adequate moisture for the microorganism to develop, but no additional free water, which makes it optimal for organisms that thrive in low-water-activity environments. It can be performed in either an inert carrier or on insoluble substrates that supply the nutrients. It resembles many valuable fungi native environments, allowing organisms to better adapt to processual conditions. Both Basidiomycetes and Ascomycetes evolved on land, and only later did some species migrate to a marine environment. The marine microorganisms of these phyla thus often prefer solid media, as most of them are isolated from sediments or solid structures and do not freely swim in the water. It also removes the need for conditions that the fungi did not evolve to be adapted to, like high shear stresses and the presence of antifoam products. This type of culture promotes secondary metabolite production—for example, coniosetin (9, Figure 3), which is a potential antibiotic from Coniochaeta ellipsoidea, can only be produced in a solid state despite the mycelium also growing well in liquid media [23][31].
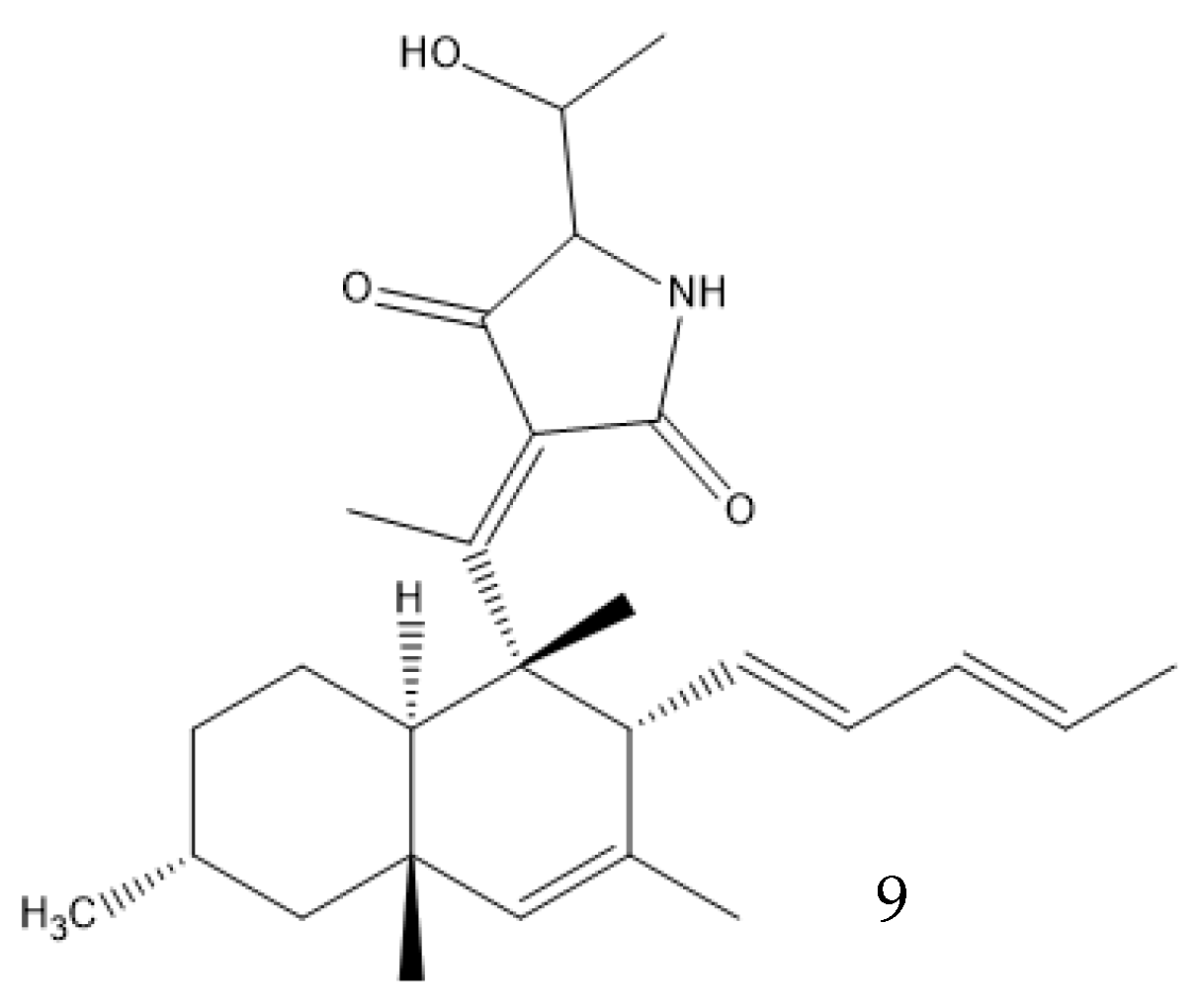
Figure 3. Coniosetin structure.
Solid-state fermentation is sometimes considered a low-technology approach, best suited to high-volume low-cost applications that require low purity like industrial enzymes—amylase, cellulase, protease, etc. However, this has been changing, and more valuable products have been produced this way, although bacteria and yeast are more commonly employed in such systems [32][33]. On a laboratory scale, it has many advantages such as higher productivity, concentration of products, and product stability (due to lower water activity), and lower catabolic repression and sterility requirements, but its use is hindered by the difficulty in scaling up [23].
2.3. Co-Culture Strategies
Co-culture or mixed fermentation strategies may be of great value to unlock the expression of cryptic pathways, producing molecules that are absent in pure-strain cultures such as pheromones, defense molecules, or metabolites that are related to symbiotic associations. These strategies consist of growing different microorganisms in the same confined environment, allowing them to interact with each other [34]. Such interactions may even alter the core metabolism of the cells, for example, by increasing the production of ergosterol [35]. In addition to a yield increase in common molecules, these types of strategies can increase the yield of previously unidentified or undetected molecules, facilitating their characterization [36]. They can also help to overcome some problems that fermentative processes undergo, including excessive metabolic burden, a lack of active enzyme expression, and by-product formation [37]. This makes sense from an ecological perspective, as the production of antibiosis molecules by a fungus is most important when exposed to a competitor [38]. A culture of Fusarium tricinctum and Streptomyces lividans produced lateropyrone (10, Figure 4) and zearalenone (11), which are two antibacterial molecules, only when co-cultivated [39]. Berkeleylactone A (12) and A26771B (13), two macrolide antibiotics, were also obtained only when two extremophilic Penicillium species—P. fuscum and P. camembertii/clavigerum—were co-cultivated [40].
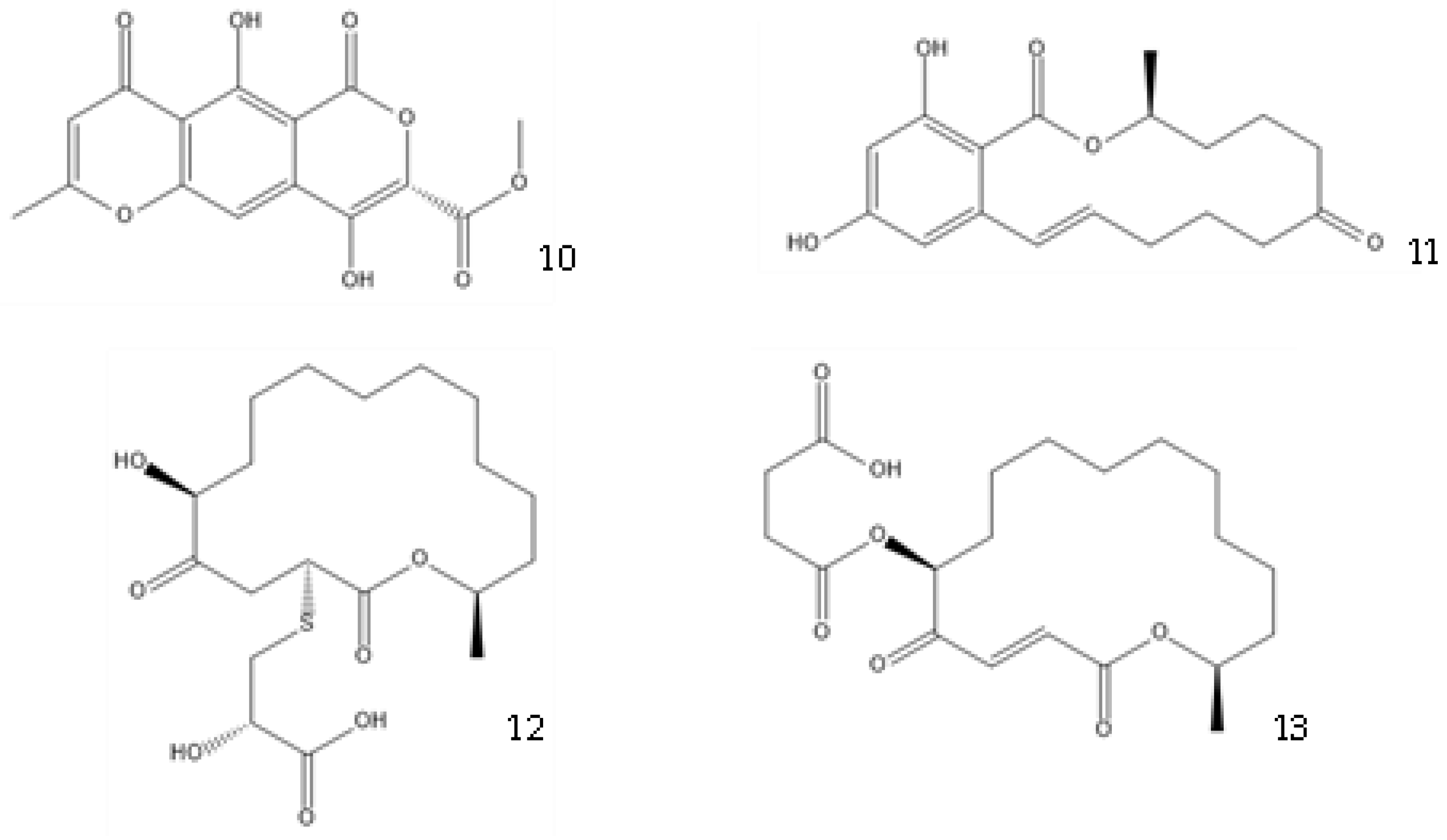
Figure 4. Structures of lateropyrone (10), zearalenone (11), berkeleylactone A (12), and A26771B (13).
To co-cultivate bacteria and fungi for drug discovery, the most common strategy is similar to regular monoculture fungi fermentations, but the liquid medium is inoculated with a small number of bacteria, often not at the same time as the fungi. Similarly, solid media like commercial rice or wheat can also be used. The bacteria should not dominate the culture but, at a small scale, can be regulated by the inoculum ratio [34][41][42]. An alternative proposed method grows the fungi in glass beads submerged in a culture medium. After the fungus has developed, the medium is replaced with a bacterial suspension, but the mycelium remains adhered to the beads, establishing a mixed culture [43]. These methods, however, have some limitations. Firstly, they are not employed more often because they require laboratory expertise in the cultivation of both fungi and bacteria [34]. Additionally, the induced metabolites that are produced are quite random. For example, when the antimicrobial activity of a culture of Streptomyces sp. (which, despite being a bacterium, has many similarities with fungi in terms of antibiotic production) was tested against human pathogens, no significant effect was found against P. aeruginosa. A co-culture with P. aeruginosa increased the 4-fold activity against all the Gram-positive bacteria tested, but, unexpectedly, did not increase the activity against that pathogen [44]. In the production steps of the drug development pipeline, especially while scaling up the reactor, it can also be difficult to keep the co-culture stable. In this regard, some methods can help to surpass this limitation, like a pulsating feed of glucose that gives a fitness advantage to alternating species. [45].
2.4. Other Optimization Strategies
Many other conditions can be varied to optimize the production of a metabolite, which are often limited by the knowledge of the mechanisms involved. Surfactants can have an interesting effect on liquid fermentation. It was found that the yield of lovastatin (14, Figure 5) production by Monascus purpureus was increased by 84.5% by the addition of Triton X-100, a nonionic surfactant. This surfactant increases the membrane permeability, facilitating the export of the product of interest and increasing the expression of the genes related to lovastatin synthesis [46][47]. The addition of Tween 80 on a Ganoderma lucidum culture, which produces bioactive exopolysaccharides (EPS), or on a Coprinopsis cinerea culture, which produces cellulose, also increased their yield [48][49].
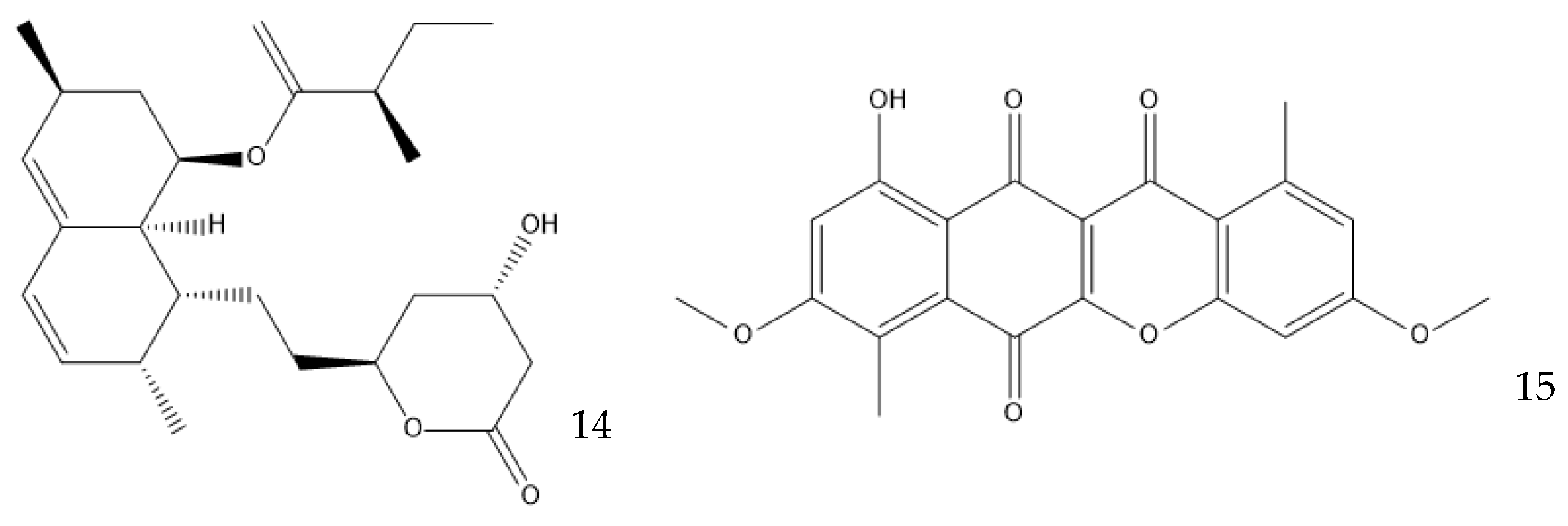
Figure 5. Structure of lovastatin (14) and bikaverin (15).
The inclusion of polymers like polyacrylic acid or sodium polyacrylate modifies the spores’ surfaces to avoid the formation of clumps and makes the mycelia grow in dispersed hyphal filaments [50]. Another exhaustive study used either XAD-16N or styrene-divinylbenzene, two resins, to induce the production of many compounds that were not present otherwise in fermentation with 349 different fungal strains [51]. Adsorptive polymeric resins can increase biomass growth and drastically change the color of the fermentation broth. They interfere with the fermentation by sequestrating components from the medium. Those might be microelements or toxic products, which are especially relevant if the product of interest is extracellular and toxic or degrades easily [52][53].
Some small molecules may serve as epigenetic modifiers by interfering with DNA methyltransferase, like 5-azacytidine and 5-aza-20-deoxycytidine, or with histone deacetylase, like compounds with hydroxamic acid or cyclic peptides (e.g., trichostatin A and trapoxin B) [52]. This may induce the expression of metabolic pathways that are cryptic in standard laboratory conditions, as those enzymes are responsible for the regulation of gene expression. Although the production of secondary metabolites is enhanced, this method is usually not specific to certain secondary metabolites. However, it was found that hydrazine boosted the production of some specific metabolites in a Dothiora sp. culture, including the antibiotic fusidic acid (8), and inhibited others [54]. Similarly, while reducing histone deacetylase activity using a mixture of compounds, Aspergillus nidulans overexpressed and under-expressed similar numbers of secondary metabolites by at least two orders of magnitude [55].
Using microparticles of talc (e.g., magnesium silicate), aluminum oxide, titanium oxide, or silver nanoparticles is another strategy to enhance the production of fungal products, specifically enzymes [56]. Talc microparticles have been shown to increase lovastatin production by Aspergillus terreus by 60%, although this effect is due to their ability to morphologically engineer the fungus. By reducing the pellet diameter, they increased the oxygen availability in the inner part of the pellets and, consequently, the product yield [57][58]. Aluminum oxide microparticles increased the amylase production by 114% in an Aspergillus oryzae fermentation through a similar mechanism [56].
There are even other parameters that can be changed to improve the production of antibiotics. Starving the cells of a nutrient can be effective in specific cases; for example, in the production of the polyketide bikaverin (15) by Fusarium oxysporum, nitrogen, phosphorous, and sulfate limitations increase the yield, while the industrial production of penicillin by P. chrysogenum must have phosphates as the limiting nutrient [19][59]. Secondary metabolites are produced in the stationary phase of cellular growth, which usually happens when there is a sugar limitation [60][61]. The pH can play a part in the production of secondary metabolites by regulating the expression of enzymes that are involved in their production. A. nidulans favors the production of penicillin in alkaline environments because the gene encoding the isopenicillin N synthetase, which is responsible for the production of an intermediate in the penicillin biosynthetic pathway, is up-regulated in these conditions [62]. This effect is observed in P. chrysogenum, but to a lesser extent [61]. Bikaverin production, on the other hand, is favored by an acidic pH [59].
Table 1 shows examples of fungal metabolites with antibacterial properties, their structure, the producing species, and the culture media used.
Table 1. Examples of fungal metabolites with antibacterial properties. Unless otherwise stated, the collected studies were relatively small-scale, conducted in liquid media, and used monoculture fermentation.
| Antibiotic | Producing Species | Structure | Media Used | Observations | References |
|---|---|---|---|---|---|
| Penicillin G | Penicillium chrysogenum | 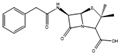 |
Glucose or molasses, 100 g/L; corn steep liquor solids, 45 g/L; phenylacetic acid, 0.65 g/L (fed continuously); vegetable oil–antifoam, 0.5 g/L; ammonium sulfate (continuously kept at 275 g/L). | Media for industrial production | [19] |
| Bis-N-norgliovictin | Asteromyces cruciatus | 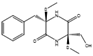 |
Glucose, 30 g/L; arginine, 1 g/L; asparagine, 2.5 g/L; glutamate, 1.5 g/L; FeSO4, 0.01 g/L; KCl, 0.5 g/L; MgSO4, 0.5 g/L; K2HPO4, 1.0 g/L; in artificial sea water. | [63] | |
| Copsin | Coprinopsis cinerea | 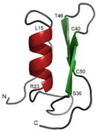 |
Glucose, 5 g/L; asparagine, 2 g/L; adenine sulfate, 50 mg/L; KH2PO4, 1 g/L; Na2HPO4, 2.3 g/L; Na2SO4, 0.3 g/L; ammonium tartrate, 0.5 g/L; thiamine-HCl, 40 μg/L; MgSO4·7H2O, 0.25 g/L; p-aminobenzoic acid, 5 mg/L. | Cultured in glass beads; co-cultured with Bacillus subtilis or Escherichia coli | [43][64] |
| Trichogin GA IV | Trichoderma longibrachiatum | 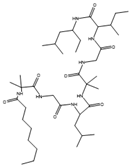 |
Glucose, 5 g/L; potassium dihydrogen phosphate, 0.8 g/L; potassium nitrate, 0.72 g/L; calcium phosphate, 0.2 g/L; magnesium sulfate, 0.5 g/L; manganese sulfate, 0.01 g/L; zinc sulfate, 0.01 g/L; copper sulfate, 0.005 g/L; iron sulfate, 0.001 g/L. | [65][66] | |
| Averufanin | Aspergillus carneus | 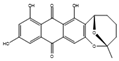 |
Glucose, 10 g/L; mannitol, 20 g/L; sucrose, 20 g/L; yeast extract, 3 g/L; corn syrup, 1 g/L; peptone, 10 g/L; tryptophan, 0.5 g/L; K2HPO4, 0.5 g/L; MgSO4·7H2O, 0.5 g/L; FeSO4·7H2O, 0.1 g/L; agar, 15 g/L. | Extracted from the solid culture medium | [67] |
| Oxasetin | Vaginatispora aquatica | 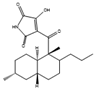 |
Extract from potato, 4 g/L; glucose, 20 g/L. pH adjusted to 7. | [68] | |
| Bovistol D | Coprinopsis strossmayeri | 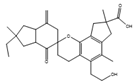 |
Extract from potato, 4 g/L; glucose, 20 g/L. | [69] | |
| Illudin I | Coprinopsis episcopalis | 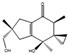 |
Sucrose, 80 g/L, yellow corn meal, 50 g/L; yeast extract, 1 g/L. | [70] | |
| Aspergicin | Two Aspergillus spp. | 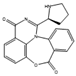 |
Glucose, 10 g/L; yeast extract, 1 g/L; peptone, 2 g/L; crude sea salt, 3.5 g/L. | Yielded another antimicrobial compound | [36] |
| Emericellin A | Emericella sp. | 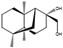 |
Peptone, 1 g/L; malt extract, 20 g/L; sucrose, 20 g/L. | Another similar compound was also isolated | [71] |
| Palmarumycin C8 | Lophiotrema sp. | 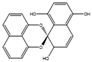 |
Glucose, 20 g/L; maltose, 10 g/L; yeast extract, 4 g/L; oatmeal, 20 g/L; 5-azacytidine, 12 mg/L. | [72] | |
| Diaporthin | Diaporthe terebinthifolii | 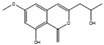 |
Malt extract, 20 g/L; glucose, 20 g/L; peptone, 1 g/L. | Other media also yielded this and another antimicrobial compound | [73] |
| Phomopsin A | Phomopsis sp. ZSU-H76 | 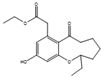 |
Glucose, 10 g/L; peptone, 2 g/L; yeast extract, 1 g/L; NaCl, 3 g/L. | Other compounds were also isolated | [74] |
| Eugenol | Neopestalotiopsis sp. MFLUCC15-1130 | 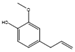 |
Extract from potato, 4 g/L; glucose, 20 g/L. | [75][76] | |
| 3-phenylpropionic acid | Cladosporium cladosporioides | 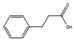 |
Extract from potato, 4 g/L; glucose, 20 g/L. | Other compounds with weaker antimicrobial effects were also identified | [77] |
The optimization of culture conditions can have an improved hit rate when using the ever-increasing available information about the biosynthetic pathways involved in the production of metabolites [78]. While some parameters should be optimized based on the producing species, others are specific to a particular pathway and must be established accordingly.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12081250
References
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180.
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459.
- Kavanagh, K. Fungi: Biology and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011.
- Stöckli, M.; Morinaka, B.I.; Lackner, G.; Kombrink, A.; Sieber, R.; Margot, C.; Stanley, C.E.; de Mello, A.J.; Piel, J.; Künzler, M. Bacteria-induced production of the antibacterial sesquiterpene lagopodin B in Coprinopsis cinerea. Mol. Microbiol. 2019, 112, 605–619.
- Zheng, H.; Kim, J.; Liew, M.; Yan, J.K.; Herrera, O.; Bok, J.W.; Kelleher, N.L.; Keller, N.P.; Wang, Y. Redox metabolites signal polymicrobial biofilm development via the NapA oxidative stress cascade in Aspergillus. Curr. Biol. 2015, 25, 29–37.
- May Zin, W.W.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inacio, A.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M.S.; et al. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97.
- Igarashi, Y.; Gohda, F.; Kadoshima, T.; Fukuda, T.; Hanafusa, T.; Shojima, A.; Nakayama, J.; Bills, G.F.; Peterson, S. Avellanin C, an inhibitor of quorum-sensing signaling in Staphylococcus aureus, from Hamigera ingelheimensis. J. Antibiot. 2015, 68, 707–710.
- Shen, B. Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr. Opin. Chem. Biol. 2003, 7, 285–295.
- Li, J.; Liu, Q.; Liu, D.; Wu, M.; Tian, C. Advances in metabolic engineering of filamentous fungi. Sheng Wu Gong Cheng Xue Bao 2021, 37, 1637–1658.
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of Linear Non-Ribosomal Peptide in Biocontrol Fungi. J. Fungi 2020, 6, 61.
- Adedeji, W.A. The treasure called antibiotics. Ann. Ib. Postgrad. Med. 2016, 14, 56–57.
- Novak, R. Are pleuromutilin antibiotics finally fit for human use? Ann. N. Y. Acad. Sci. 2011, 1241, 71–81.
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Zaher, A.M.; Makboul, M.A.; Moharram, A.M.; Tekwani, B.L.; Calderón, A.I. A new enniatin antibiotic from the endophyte Fusarium tricinctum Corda. J. Antibiot. 2015, 68, 197–200.
- Dobie, D.; Gray, J. Fusidic acid resistance in Staphylococcus aureus. Arch. Dis. Child. 2004, 89, 74.
- Frisvad, J.C. Media and growth conditions for induction of secondary metabolite production. In Fungal Secondary Metabolism; Humana Press: Totowa, NJ, USA, 2012; pp. 47–58.
- Ariantari, N.P.; Daletos, G.; Mándi, A.; Kurtán, T.; Müller, W.E.; Lin, W.; Ancheeva, E.; Proksch, P. Expanding the chemical diversity of an endophytic fungus Bulgaria inquinans, an ascomycete associated with mistletoe, through an OSMAC approach. RSC Adv. 2019, 9, 25119–25132.
- Paranagama, P.A.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Uncovering biosynthetic potential of plant-associated fungi: Effect of culture conditions on metabolite production by Paraphaeosphaeria quadriseptata and Chaetomium chiversii. J. Nat. Prod. 2007, 70, 1939–1945.
- Shuler, M.L.; Kargı, F. Bioprocess Engineering: Basic Concepts, 2nd ed.; Pearson New International Edition, Ed.; Pearson Education Limited: Harlow, Essex, 2014.
- Menezes, J.C.; Alves, T.P.; Cardoso, J.P. Biotecnologia microbiana: A produção de penicilina. In Biotecnologia: Fundamentos e Aplicações, 1st ed.; Lima, N., Mota, M., Eds.; DIFEL: São Paulo, Brazil, 2000; pp. 78–95.
- Nielsen, J.; Johansen, C.L.; Jacobsen, M.; Krabben, P.; Villadsen, J. Pellet formation and fragmentation in submerged cultures of Penicillium chrysogenum and its relation to penicillin production. Biotechnol. Prog. 1995, 11, 93–98.
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006.
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186.
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486.
- Zhang, B.-B.; Guan, Y.-Y.; Hu, P.-F.; Chen, L.; Xu, G.-R.; Liu, L.; Cheung, P.C.K. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554.
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Elsevier: Amsterdam, The Netherlands, 2017.
- Gibbs, P.; Seviour, R.; Schmid, F. Growth of filamentous fungi in submerged culture: Problems and possible solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48.
- Riley, G.; Tucker, K.; Paul, G.; Thomas, C. Effect of biomass concentration and mycelial morphology on fermentation broth rheology. Biotechnol. Bioeng. 2000, 68, 160–172.
- Ibrahim, D.; Weloosamy, H.; Lim, S.-H. Effect of agitation speed on the morphology of Aspergillus niger HFD5A-1 hyphae and its pectinase production in submerged fermentation. World J. Biol. Chem. 2015, 6, 265.
- Purwanto, L.; Ibrahim, D.; Sudrajat, H. Effect of agitation speed on morphological changes in Aspergillus niger hyphae during production of tannase. World J. Chem. 2009, 4, 34–38.
- Segeth, M.P.; Bonnefoy, A.; Broenstrup, M.; Knauf, M.; Schummer, D.; Toti, L.; Vertesy, L.; Wetzel-Raynal, M.-C.; Wink, J.; Seibert, G. Coniosetin, a novel tetramic acid antibiotic from Coniochaeta ellipsoidea DSM 13856. J. Antibiot. 2003, 56, 114–122.
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84.
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566.
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521.
- Nguyen, P.-A.; Strub, C.; Lagrée, M.; Bertrand-Michel, J.; Schorr-Galindo, S.; Fontana, A. Study of in vitro interaction between Fusarium verticillioides and Streptomyces sp. using metabolomics. Folia Microbiol. 2020, 65, 303–314.
- Zhu, F.; Chen, G.; Chen, X.; Huang, M.; Wan, X. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769.
- Jones, J.A.; Wang, X. Use of bacterial co-cultures for the efficient production of chemicals. Curr. Opin. Biotechnol. 2018, 53, 33–38.
- Karwehl, S.; Stadler, M. Exploitation of fungal biodiversity for discovery of novel antibiotics. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Stadler, M., Dersch, P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 303–338.
- Moussa, M.; Ebrahim, W.; Bonus, M.; Gohlke, H.; Mándi, A.; Kurtán, T.; Hartmann, R.; Kalscheuer, R.; Lin, W.; Liu, Z.; et al. Co-culture of the fungus Fusarium tricinctum with Streptomyces lividans induces production of cryptic naphthoquinone dimers. RSC Advances 2019, 9, 1491–1500.
- Stierle, A.A.; Stierle, D.B.; Decato, D.; Priestley, N.D.; Alverson, J.B.; Hoody, J.; McGrath, K.; Klepacki, D. The berkeleylactones, antibiotic macrolides from fungal coculture. Journal of Natural Products 2017, 80, 1150–1160.
- Chen, H.; Daletos, G.; Abdel-Aziz, M.S.; Thomy, D.; Dai, H.; Brötz-Oesterhelt, H.; Lin, W.; Proksch, P. Inducing secondary metabolite production by the soil-dwelling fungus Aspergillus terreus through bacterial co-culture. Phytochem. Lett. 2015, 12, 35–41.
- Chen, T.; Zhou, Y.; Lu, Y.; Zhang, H. Advances in heterologous biosynthesis of plant and fungal natural products by modular co-culture engineering. Biotechnol. Lett. 2019, 41, 27–34.
- Essig, A.; Hofmann, D.; Münch, D.; Gayathri, S.; Künzler, M.; Kallio, P.T.; Sahl, H.-G.; Wider, G.; Schneider, T.; Aebi, M. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 2014, 289, 34953–34964.
- Sung, A.A.; Gromek, S.M.; Balunas, M.J. Upregulation and identification of antibiotic activity of a marine-derived Streptomyces sp. via co-cultures with human pathogens. Mar. Drugs 2017, 15, 250.
- Martinez, J.A.; Delvenne, M.; Henrion, L.; Moreno, F.; Telek, S.; Dusny, C.; Delvigne, F. Controlling microbial co-culture based on substrate pulsing can lead to stability through differential fitness advantages. PLoS Comput. Biol. 2022, 18, e1010674.
- Zhang, J.; Wang, Y.L.; Lu, L.P.; Zhang, B.B.; Xu, G.R. Enhanced production of Monacolin K by addition of precursors and surfactants in submerged fermentation of Monascus purpureus 9901. Biotechnol. Appl. Biochem. 2014, 61, 202–207.
- Yang, X.; Xiang, L.; Zhang, C.; Cao, Y.; Wang, C. Promotion of monacolin K production in Monascus extractive fermentation: The variation in fungal morphology and in the expression levels of biosynthetic gene clusters. J. Sci. Food Agric. 2021, 101, 5652–5659.
- Yang, X.; Yang, Y.; Zhang, Y.; He, J.; Xie, Y. Enhanced exopolysaccharide production in submerged fermentation of Ganoderma lucidum by Tween 80 supplementation. Bioprocess Biosyst. Eng. 2021, 44, 47–56.
- Maan, P.; Sengar, R. Standardization of Nutrient Salt Solution for Improved Production of Cellulase from C. cinerea. Biotech Today Int. J. Biol. Sci. 2017, 7, 35–40.
- Jones, P.; Shahab, B.A.; Trinci, A.P.J.; Moore, D. Effect of polymeric additives, especially Junlon and Hostacerin, on growth of some basidiomycetes in submerged culture. Trans. Br. Mycol. Soc. 1988, 90, 577–583.
- González-Menéndez, V.; Crespo, G.; De Pedro, N.; Diaz, C.; Martín, J.; Serrano, R.; Mackenzie, T.A.; Justicia, C.; González-Tejero, M.R.; Casares, M. Fungal endophytes from arid areas of Andalusia: High potential sources for antifungal and antitumoral agents. Sci. Rep. 2018, 8, 9729.
- Venugopalan, A.; Srivastava, S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015, 33, 873–887.
- Luo, H.; Liu, H.; Cao, Y.; Xu, D.; Mao, Z.; Mou, Y.; Meng, J.; Lai, D.; Liu, Y.; Zhou, L. Enhanced production of botrallin and TMC-264 with in Situ macroporous resin adsorption in mycelial liquid culture of the endophytic fungus Hyalodendriella sp. Ponipodef12. Molecules 2014, 19, 14221–14234.
- González Menéndez, V.M. Enhancement of Chemical Diversity in Fungal Endophytes from Arid Plants of Andalusia. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2019.
- Albright, J.C.; Henke, M.T.; Soukup, A.A.; McClure, R.A.; Thomson, R.J.; Keller, N.P.; Kelleher, N.L. Large-scale metabolomics reveals a complex response of Aspergillus nidulans to epigenetic perturbation. ACS Chem. Biol. 2015, 10, 1535–1541.
- Singh, B. Engineering fungal morphology for enhanced production of hydrolytic enzymes by Aspergillus oryzae SBS50 using microparticles. 3 Biotech 2018, 8, 283.
- Boruta, T.; Bizukojc, M. Application of aluminum oxide nanoparticles in Aspergillus terreus cultivations: Evaluating the effects on lovastatin production and fungal morphology. BioMed Res. Int. 2019, 2019, 5832496.
- Gonciarz, J.; Bizukojc, M. Adding talc microparticles to Aspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Eng. Life Sci. 2014, 14, 190–200.
- Limón, M.C.; Rodríguez-Ortiz, R.; Avalos, J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29.
- Sánchez, S.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Ávalos, M.; Guzmán-Trampe, S.; et al. Carbon source regulation of antibiotic production. J. Antibiot. 2010, 63, 442–459.
- Martín, J.F. Molecular control of expression of penicillin biosynthesis genes in fungi: Regulatory proteins interact with a bidirectional promoter region. J. Bacteriol. 2000, 182, 2355–2362.
- Espeso, E.A.; Tilburn, J.; Arst, H., Jr.; Penalva, M. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 1993, 12, 3947–3956.
- Gulder, T.A.M.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.F.; Gross, H. Isolation, Structure Elucidation and Total Synthesis of Lajollamide A from the Marine Fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935.
- Franzoi, M.; van Heuvel, Y.; Thomann, S.; Schürch, N.; Kallio, P.T.; Venier, P.; Essig, A. Structural insights into the mode of action of the peptide antibiotic copsin. Biochemistry 2017, 56, 4992–5001.
- Auvin-Guette, C.; Rebuffat, S.; Prigent, Y.; Bodo, B. Trichogin A IV, an 11-residue lipopeptaibol from Trichoderma longibrachiatum. J. Am. Chem. Soc. 1992, 114, 2170–2174.
- De Zotti, M. Trichogin GA IV: An antibacterial and protease-resistant peptide TRICHOGIN GA IV. J. Pept. Sci. 2009, 15, 615–619.
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tansel Tanrıkul, T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.; Zou, K.; Liu, Z.; Proksch, P. Induction of new metabolites from sponge-associated fungus Aspergillus carneus by OSMAC approach. Fitoterapia 2018, 131, 9–14.
- He, H.; Janso, J.E.; Yang, H.Y.; Singh, M.P.; Bernan, V.S.; Greenstein, M.; Carter, G.T. Oxasetin, a new antibacterial polyketide produced by fungus Vaginatispora aquatica, HK1821. J. Antibiot. 2002, 55, 821–825.
- Banks, A.M.; Song, L.; Challis, G.L.; Bailey, A.M.; Foster, G.D. Bovistol B, bovistol D and strossmayerin: Sesquiterpene metabolites from the culture filtrate of the basidiomycete Coprinopsis strossmayeri. PLoS ONE 2020, 15, e0229925.
- Reina, M.a.; Orihuela, J.C.; González-Coloma, A.; de Inés, C.; de la Cruz, M.; González del Val, A.; Torno, J.R.; Fraga, B.M. Four illudane sesquiterpenes from Coprinopsis episcopalis. Phytochemistry 2004, 65, 381–385.
- Pang, X.-J.; Zhang, S.-B.; Xian, P.-J.; Wu, X.; Yang, D.-F.; Fu, H.-Y.; Yang, X.-L. Emericellins A and B: Two sesquiterpenoids with an unprecedented tricyclohendecane scaffold from the liquid cultures of endophytic fungus Emericella sp. XL 029. Fitoterapia 2018, 131, 55–58.
- Gakuubi, M.M.; Ching, K.C.; Munusamy, M.; Wibowo, M.; Liang, Z.-X.; Kanagasundaram, Y.; Ng, S.B. Enhancing the discovery of bioactive secondary metabolites from fungal endophytes using chemical elicitation and variation of fermentation media. Front. Microbiol. 2022, 13, 898976.
- de Medeiros, A.G.; Savi, D.C.; Mitra, P.; Shaaban, K.A.; Jha, A.K.; Thorson, J.S.; Rohr, J.; Glienke, C. Bioprospecting of Diaporthe terebinthifolii LGMF907 for antimicrobial compounds. Folia Microbiol. 2018, 63, 499–505.
- Huang, Z.; Cai, X.; Shao, C.; She, Z.; Xia, X.; Chen, Y.; Yang, J.; Zhou, S.; Lin, Y. Chemistry and weak antimicrobial activities of phomopsins produced by mangrove endophytic fungus Phomopsis sp. ZSU-H76. Phytochemistry 2008, 69, 1604–1608.
- Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Pripdeevech, P. Production of eugenol from fungal endophytes Neopestalotiopsis sp. and Diaporthe sp. isolated from Cinnamomum loureiroi leaves. PeerJ 2019, 7, e6427.
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671.
- Yehia, R.S.; Osman, G.H.; Assaggaf, H.; Salem, R.; Mohamed, M.S.M. Isolation of potential antimicrobial metabolites from endophytic fungus Cladosporium cladosporioides from endemic plant Zygophyllum mandavillei. S. Afr. J. Bot. 2020, 134, 296–302.
- Udwary, D.W.; Otani, H.; Mouncey, N.J. New keys to unlock the treasure trove of microbial natural products. Nat. Rev. Microbiol. 2021, 19, 683.
This entry is offline, you can click here to edit this entry!
