Polypoidal choroidal vasculopathy (PCV) was generally considered as a subtype of age-related macular degeneration. Currently, the gold standard for diagnosing PCV is indocyanine green angiography (ICGA). However, it has been concerned about its rare but deadly complications and inconvenience during clinical visits. ICGA cannot provide detailed anatomical information about the disease lesions, either. Swept-source optical coherence tomography angiography (SS-OCTA) is a new, noninvasive, fast, and high-quality imaging method for PCV, which is significantly improved in many aspects compared with the previous spectral domain OCTA (SD-OCTA). With SS-OCTA, the lesion characteristics of PCV have been extensively studied. SS-OCTA revealed a new morphological pattern of the polypoidal lesions (PLs) call “tangled vasculature”, suggesting the neovascular essence of PLs, instead of aneurysmal dilations. In addition, by assessing the changes of choroidal structure, such as the choriocapillaris flow voids, the hypothetical developing model of PCV has been proposed. Due to the improved detecting ability, SS-OCTA might also play a more important role in differential diagnosis, disease reactivation monitoring in the future. This article reviewed recently published papers that focused on imaging PCV with SS-OCTA, summarized advances in the presentation and relevant new pathogenic findings of PCV, and assessed its value in the clinical management of PCV as described above, hoping to update current understandings of PCV and suggest directions for future studies.
1. Presentation of Polypoidal Lesions on SS-OCTA
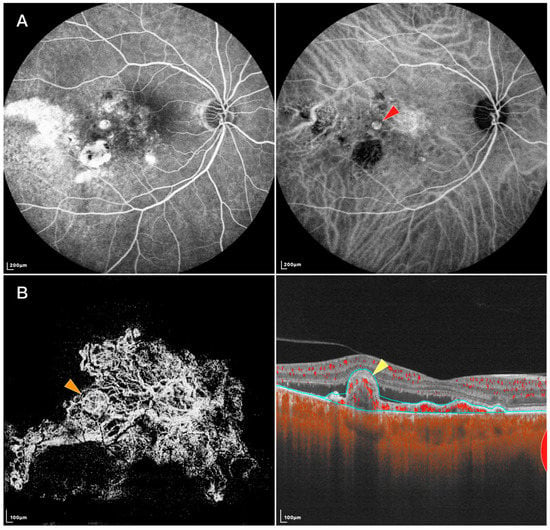
Using SS-OCTA, a study found the shape of PLs resembled those in early ICGA phases. Other studies described PLs’ appearance on SS-OCTA as round hyper-reflective lesions with or without hypo-reflective outlines or as ring-like hyper-reflective structures, which was in accordance with previous studies using ICGA or SD-OCTA [
27,
28,
29]. However, SS-OCTA recently identified a new PL pattern called “tangled vasculature” or “tangled vessel” (shown in
Figure 1) [
30,
31,
32,
33]. Bo et al. [
30] found that tangled vessels appeared to derive from existing BVNs and were arranged in ring, whorl, or cluster patterns. Thus, those round or ring-like structures on SD-OCTA might be different layouts of tangled neovascularization. Yuzawa et al. also noted the tangled vessels were visible as a ring of hyper-fluorescence on ICGA as the dye intensity faded from the central lumens during washout [
34]. The previous belief that PLs were aneurysmal dilations was based on the phenomenon of dye washout and turbulent blood flow on ICGA, but these features could also be found within a tangled vascular structure [
35,
36]. Rebhun et al. [
10] further developed an OCTA algorithm called variable interscan time analysis (VISTA) to display relative blood flow speeds in the retinal and choroidal vasculatures. Using SS-OCTA VISTA, they demonstrated non-uniform blood flow speeds within a single PL, indicating different vascular calibers and directions exist within the tangled vasculatures [
30]. Other evidence includes: (1) Clinicopathological investigations have shown that PLs of PCV were vascular in nature, while the single aneurysmal structure has not been detected in most histopathologic examinations [
37,
38,
39]; (2) Aneurysms should not respond to anti-vascular endothelial growth factor (VEGF) treatment, whereas neovascularization is likely to respond to it. Indeed, PLs disappeared or decreased in size and complexity after anti-VEGF therapies [
30,
40,
41]. However, this hypothesis has difficulty explaining the phenomenon of PL pulsation observed on ICGA, indicating the nature of PLs might be heterogeneous. Indeed, some PLs lack tangled presentations on SS-OCTA. A recent clinicopathological study proposed PLs to be abnormalities of the Bruch’s membrane. The lesions are characterized by Bruch’s membrane schisis, which is filled with serosanguineous materials. As dye accumulates in a schisis space, it may appear as a “polyp-like” structure on ICGA [
42]. Thus, further studies investigating the essence of the lesion and associated pathology are needed to verify the structural diversity of PLs.
Figure 1. FFA, IGCA, and SS-OCTA images of an eye with PCV: (A) FFA (left) and ICGA (right) of the PCV lesion. Red arrowhead: nodular-shaped PL; (B) En face (left) and B-scan (right) SS-OCTA of the same lesion. Orange arrowhead: tangled vascular structure corresponding to the PL on ICGA. Yellow arrowhead: RPE protrusion and ring-like structure under RPE corresponding to the tangled vascular structure on en face OCTA.
Regarding pathogenesis, Wang et al. found small dome-shaped pigment epithelial detachments (PEDs) that were often ignored on SD-OCT showed vascularized PEDs and corresponded with cluster-like structures at the edge of BVNs on en face SS-OCTA. They inferred the formation of some PLs might be the twisted ends of new vessels, tangling into a larger cluster-like structure and protruding into the retina [
43]. Previous studies have reported that PLs might develop with the accumulation of serous PEDs. PLs tend to be located at the margin of serous PEDs and then detach from Bruch’s membrane as increasing fluid infiltrates under the PLs [
44]. Indeed, B-scan SS-OCTA could visualize PLs attached to Bruch’s membrane with or without serous PEDs and PLs detached from Bruch’s membrane with serous PEDs [
31]. Although far from revealing the initiation of PCV formation, these findings might provide an overview of the later development stage.
2. Presentation of Branching Vascular Networks on SS-OCTA
Since the manifestation of BVNs has been well defined using SD-OCTA, SS-OCTA generally attained similar results in characterizing their morphology and locality. It has been reported that the measured BVN areas of treatment-naive PCV were not statistically different between ICGA and SS-OCTA. SS-OCTA could even delineate the margins of BVNs better when their sizes were small [
28]. Allowing some PLs to be recognized as tangled vascular structures associated with BVNs, might also help understand the role of neovascularization in the etiology of PCV and possible implications in treatment. In addition, investigators previously used SD-OCTA to classify BVNs into different subtypes based on their morphological features, where these classification systems showed some consistency with those based on ICGA and fundus fluorescence angiography (FFA) in predicting visual prognosis [
45,
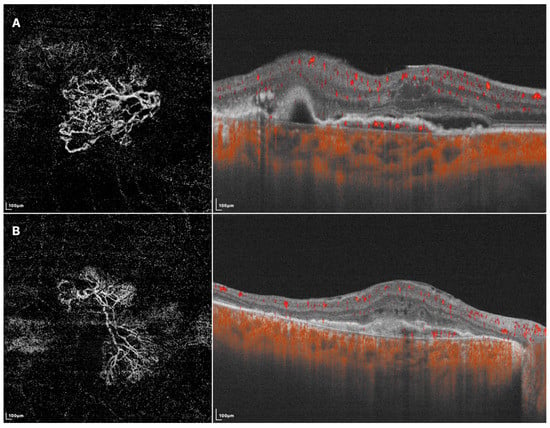
46]. Using SS-OCTA, BVNs can be described with more morphological patterns, such as dense, very dense (bush-shaped), loose (dead-tree-shaped), pseudopod-like, and anastomosing forms. Patients with loose patterns had a higher ratio of active disease compared with those with dense patterns (Shown in
Figure 2) [
29,
47]. A recent study classified BVNs into three morphological types (“trunk”, “glomeruli”, and “stick” type) and revealed significant differences among BVN types regarding lesion structural characteristics, such as PED area, subretinal fluid area, and BVN area, though the visual acuity at 12 months after anti-VEGF therapy was similar [
48]. These results showed the potential of OCTA in guiding accurate and personalized management of PCV in the future. More investigations are required before they become clinically meaningful, and SS-OCTA can help accelerate this course of investigation.
Figure 2. SS-OCTA images of two different BVN patterns that might indicate active (A) or quiescent (B) lesions. (A) En face SS-OCTA (left) shows a loose pattern with characteristics such as anastomosis and loops. The corresponding B-scan SS-OCTA (right) shows sub-retinal fluid and rich flow signals under RPE; (B) En face SS-OCTA (left) shows a dense pattern with characteristics such as long and filamentous shapes and lack of loops. The corresponding B-scan SS-OCTA (right) shows a lack of intra/sub-retinal fluid and flow signals under RPE.
3. Choroidal Changes of PCV on SS-OCTA
PCV is now considered to belong among pachychoroid diseases because of its abnormally increased choroidal thickness, which was noticed using SS-OCT [
23,
49]. Lee et al. further found that PCV lesion sites commonly featured pachyvessels and a decreased ratio of choriocapillaris/Sattler layer to total choroidal thickness. They inferred that the expansion of the outer choroidal vessels might cause mechanical damage to the Bruch’s membrane-RPE complex, and the attenuation of choriocapillaris could provide a relatively ischemic environment, leading to the expression of angiogenic factors [
50]. The choroidal vascular index, defined as the ratio of the luminal area over the total choroidal area, was found to be lower in PCV compared to normal eyes [
15,
51,
52]. A possible explanation is that the increased choroidal hyperpermeability in PCV eyes may increase choroidal stroma volume and thus decrease the choroidal vascular index [
53]. Similarly, SS-OCTA revealed that after anti-VEGF treatment, decreases in PED volumes correlated with decreases in mean choroidal thickness as well as increases in choroidal vascular index measurements. Hence, the PCV lesion was proposed to serve as a high-volume arteriovenous shunt from the impaired choroidal circulation, consequently causing transudation into the choroidal stroma, which leads to changes in choroidal thickness and choroidal vascular index, as well as the corresponding response after anti-VEGF treatment [
54]. These studies further highlighted the role of pachychoroid features in the pathogenesis of PCV.
Investigating the fellow eyes of unilateral PCV patients may aid in understanding the early changes of this disease since PCV-unaffected fellow eyes sometimes also exhibit thick choroidal features [
23,
55,
56]. Previous studies based on SD-OCTA have found no difference in choriocapillaris flow density between the fellow eyes of PCV and normal eyes [
57]. Another biomarker used for evaluating the choriocapillaris is the choriocapillaris flow void (FV), which refers to the part of the choriocapillaris slab without blood flow signals. Kamei et al. concluded that the fellow eyes of PCV and typical AMD did not exhibit significant differences in FV area, individual FV size, number of FVs, or vascular diameter index [
58]. However, Wu et al. recently reported that when FVs were grouped by size (ranging from 400 to 1125 μm
2) and assessed using SS-OCTA, the number of small-sized FVs (under 750 μm
2) substantially decreased in PCV fellow eyes compared to the normal group. They suggested that this difference was due to the relatively larger number and smaller size of these FVs, so their decrease may not significantly impact the total number and average size of all FVs [
55,
59]. Another study based on SS-OCTA also reported the mean choriocapillaris FV was highest in eyes with PCV, followed by fellow unaffected eyes, and lowest in normal eyes in 1 mm and 1.5 mm area [
60]. These findings support the notion that choriocapillaris impairment may be an early change of pachychoroid spectrum diseases [
61]. However, it remains unclear whether the attenuation of choriocapillaris is a primary process or secondary to the dilation of the outer choroid. Further studies are needed to explore the relationship between choriocapillaris damage and pachyvessels, as well as the underlying mechanism.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13142458