Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Endocrine disruptor chemicals (EDCs) can have a harmful effect on the human body’s endocrine system and thus adversely affect the development, reproduction, neurological, cardiovascular, and immune systems and metabolism in humans and wildlife. Bisphenol A (BPA) is a proven EDC capable of mimicking or blocking receptors and altering hormone concentrations and metabolism. Although consumed in low doses, it can stimulate cellular responses and affect the body’s functions. In humans, exposure to BPA has been correlated with the onset or development of several diseases.
- bisphenol A
- endocrine disruptor
- reproductive toxicity
- infertility
- male reproduction
1. Introduction
Endocrine disruptor chemicals (EDC) can have a harmful effect on the endocrine system [1]. They are defined by the Environmental Protection Agency of the United States of America as “an agent that interferes with the synthesis, secretion, transportation, binding or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behaviour” [2][3]. By interfering with the hormonal system, they adversely affect the development, reproduction, neurological, cardiovascular, immune, and metabolism systems in both humans and wildlife. These effects can be observed after long exposure and may also have consequences for the next generation, i.e., they may vary depending on the time of exposure and the hormonal balance of the exposed individual, which is determined by age, sex, and other factors [1].
According to the World Health Organization (WHO), EDs are mostly man-made and are found in various materials such as pesticides, metals, and additives or contaminants in food and personal care products. Human exposure occurs through ingesting food, dust, and water and inhaling gases and particles in the air and through the skin. There may also be passage to the foetus through the placenta and to children through breast milk [1]. More specifically, adults come into contact mainly through ingestion of contaminated drinking water, meat, and fatty dairy products and inhalation of polluted air, while babies are exposed through breastfeeding, contact with baby products, and also by inhalation of polluted air [2].
EDs are highly heterogeneous and can be classified as natural and synthetic or grouped according to their origin [2][4]. They are also considered “chemical chameleons”, i.e., they have different mechanisms of action depending on the value of their concentration and the stage of development of the affected tissue [5]. More concrete examples of these endocrine disruptors are diethylstilbestrol, Bisphenol A, phthalates, polybrominated diphenyl ethers, and parabens, among others [1].
Bisphenol A (BPA) is an endocrine disruptor capable of mimicking or blocking receptors and altering hormone concentrations and their metabolism. Despite being consumed in low doses, it can stimulate cellular responses and affect body functions [6]. In humans, increased BPA levels correlate with the onset or development of various diseases, health problems, and medical conditions [7]. It can have several effects throughout the body and at different stages of the life cycle [8].
BPA seems to cause multi-systemic and multi-organ toxicity in animal models; however, several questions about the effects of exposure in humans still need to be answered, and more research is needed [9]. Thus, this substance represents an important challenge for the current industrialised society, requiring an assessment of the overall impact on human health since we are continuously exposed to this compound.
The available literature points out that the reproductive system may be one of the main targets of BPA toxicity, which manages to interfere negatively with male fertility.
2. Sources of Exposure
The degree of exposure to BPA can vary dramatically depending on various socioeconomic factors, lifestyle, comorbidities, and routes of exposure, like food, water, air, and soil (Figure 1) [10].
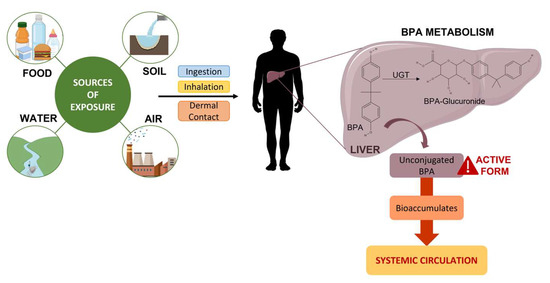
Figure 1. Sources of exposure and consequent metabolism of BPA.
2.1. Food
The main source of BPA contamination in humans is through food [9][11][12]. Its presence in edible products is related to the exposure of animals and raw materials to BPA, the accumulation of BPA in the environment, and the contact of food with polymers containing this substance [10]. Direct contamination of food is caused by the migration of BPA present in the containers in which food is stored. Epoxy resins produced with BPA are used as a coating on the surfaces of metal cans that are in contact with food and beverages, while polycarbonate plastics are used in containers that transport/store food and beverages [11]. The European Food Safety Authority (EFSA) has recently updated the tolerable daily intake of BPA from 4 μg to 0.2 ng per kilogram of body weight per day [13][14]. The previous TDI was temporary due to uncertainties in the evidence, whereas the revised EFSA decision was made according to new scientific evidence that established BPA as a health risk concern since, in all age groups, average and high exposure to BPA exceeded the 0.2 ng per kilogram of body weight per day [14]. On the other hand, the Environmental Protection Agency of the United States of America sets the tolerable value daily at 50 μg/kg/day [15]. Moreover, according to the European Commission, the migration of BPA into food from varnishes or coatings applied to materials and objects should not exceed a specific migration limit of 0.05 mg of BPA per kilogram of food. However, the migration of BPA is not permitted from varnishes or coatings applied to materials and articles specifically intended to be in contact with infant formulae, processed cereal-based foods, baby foods, special medical foods developed to meet the nutritional requirements of infants and young children, and milk beverages and similar products specifically intended for young children, as referred to in Regulation (EU) No 609/2013 [13][14]. BPA can migrate from plastics after washing and sterilisation in alkaline solutions or in hot water (e.g., steam) [11]. The migration ratio of BPA from polycarbonate bottles to other solutions varies according to their chemical properties. Temperature and, particularly, the prolonged use of bottles increases the hydrolysis of the polymer, causing a more intense migration to water [10]. BPA is authorised to be used as a monomer in plastic food contact materials in accordance with Commission Regulation (EU) No 10/2011. In addition, Commission Implementing Regulation (EU) No 321/2011, which amends the abovementioned agreement, imposes a restriction on the use of BPA in the manufacture of baby bottles for children, in accordance with the precautionary principle [13][14].
2.2. Water
BPA can be found in the aquatic environment and has been detected at high levels in leachates from landfills or factories, especially those that manufacture and process plastics [11]. In surface waters, it is usually found in low concentrations; however, near waste deposits contaminated with this substance or waste deposits of plastic materials, the concentrations are much higher [10]. BPA can also be detected in river waters; it can be degraded in aerobic conditions but not in anaerobic conditions. Despite the degradation of BPA in freshwater by bacteria and sunlight, its half-life of 3 to 5 days may be sufficient to cause harmful effects on aquatic organisms due to the metabolites that persist. Interestingly, BPA can persist longer in seawater than in river water without any degradation (about 30 days), and the bio-available fraction of dissolved BPA can increase with salinity. Thus, the possibility of contamination of a marine organism may be greater than a freshwater organism. Therefore, from the aquatic environment, the ingestion of freshwater fish or seafood contaminated with BPA may be the main route of contamination for humans [11][16]. Bacteria and other microorganisms that effectively degrade this substance may be useful in its removal from the polluted environment [10].
2.3. Air
Inhalation is the second main source of exposure [9]. BPA is emitted into the atmosphere during industrial activity, where it can remain due to its adhesion to particulate matter. The possibility of inhaling high levels of BPA through the air is very low [6][10][11]. However, due to the large quantities produced annually, workers in companies engaged in the production of plastics are an exception [17]. Various household items manufactured from BPA, such as epoxy-based flooring and electronic equipment, can release and volatilise this compound with prolonged use. Consequently, BPA can accumulate in household dust and be inhaled [9].
2.4. Soil
The main source of BPA in soils is the terrestrial application of sewage or bio-solid sludge and human waste. BPA released into groundwater or surface water can be absorbed into soil or sediment. It was found that there are higher levels of BPA in sediments than in surface waters. However, soil contamination can be correlated with population density due to the increase in human waste contaminated with BPA, such as household and/or industrial waste [11][16].
BPA is thought to have a moderate affinity with soil organic matter and thus is unlikely to be mobile or bio-available in soils. However, mobility can be affected by soil chemistry and texture. There is evidence of an increase in BPA sorption in the presence of iron, cadmium, and lead and rapid and complete desorption in sandy and acidic soils [16].
3. Conjugation, Metabolism, and Excretion
In humans, orally ingested BPA is rapidly and efficiently absorbed through the gastrointestinal tract (>95% of the dose) and subsequently metabolised in the liver, which plays an essential role in the metabolism of this substance (Figure 1) [8][9][18]. Its glucuronidation, the main biotransformation pathway, is a detoxification reaction that is carried out by uridine diphosphate glycuronosyltransferase (UGT), a glycosyltransferase that is present in the liver. UGT in the human foetal liver is present at a concentration five times lower than in the adult liver; this means that a foetus may face a higher risk of deleterious effects [9][11]. This process increases the solubility of BPA in water, which leads to faster excretion in the urine (half-life of 5.4–6.4 h) [19].
BPA glucuronide, with little or no estrogenic activity, is the main metabolite found in urine, while free or unconjugated BPA, considered the active form, is the main component present in faeces [11][18]. More than 99% of free BPA and its metabolites are excreted in faeces and urine, and less than 1% accumulates in tissues [11]. Some studies suggest that there may be gender differences in metabolite concentrations in the urine, with women having higher levels of BPA sulphate and men having higher levels of BPA glucuronide [8][11].
BPA is also conjugated in vivo in BPA sulphate by sulfotransferases found in the liver. BPA sulfation eliminates its estrogenic activity [8][18].
However, unlike dietary exposure, almost all BPA resulting from transdermal exposure prevents hepatic metabolism, resulting in significantly higher concentrations of the unconjugated form (free BPA) in the bloodstream [19].
Detection in Biological Samples
Given the prevalence of BPA in our environment, it is not surprising that measurable levels have been detected in most individuals who were analysed. It is possible to detect the presence of BPA in human tissues and fluids, including maternal blood (0.3–18.9 ng/mL), maternal urine (31.9 μg/L), amniotic fluid (median = 0.26 ng/mL), placental tissue (median = 12.7 ng/g), umbilical cord blood (0.2–9.2 ng/mL), breast milk (0.61–0.7 μg/L), and human colostrum (3.41 ng/mL) [7][10][17]. Concentrations of unconjugated BPA in human serum have been detected at levels ranging from 0.2 to 20 ng/mL [17]. Depending on the pathway by which the individual is exposed, BPA levels in the circulation may also be different [8]. As most of the BPA consumed orally is excreted in the urine (concentration varies between 1.6 and 946 μg/L) in less than 24 h after consumption, and the expected concentrations in the blood are very low, urine is the preferred body fluid for estimating BPA exposure in humans [18].
Several studies have examined BPA levels in the serum of pregnant women, umbilical cord blood, and foetal plasma. The results of these studies indicate that this substance crosses the placental barrier [20]. Of particular concern are the high levels detected mainly in developmental stages with a possible higher sensitivity to BPA [17]. BPA can accumulate in foetuses due to lower metabolic clearance. After swallowing amniotic fluid, BPA can be conjugated by the foetal liver. Thus, a foetus is susceptible to BPA exposure throughout development and may be exposed to even higher levels than those measured in the adult’s blood. Another important consideration for the health of the newborn is the potential exposure to BPA in breast milk since it is a compound with lipophilic properties [17]. By being lipophilic, it can accumulate in adipose tissue, including fat mammary glands, and cause dysregulation in various metabolic processes, both in the baby and in the adult [9][10].
4. Mechanisms of Action
BPA, with a structure similar to 17β-oestradiol (E2), binds to both the alpha (ERα) and the beta oestrogen receptors (ERβ), with approximately 10 times more affinity for ERβ. This EDC can also bind to the G-protein-coupled oestrogen receptor (GPER), the gamma peroxisome proliferator-activated receptor (PPAR-γ), and the orphan nuclear oestrogen gamma receptor (ERR-γ) [21]. Binding to these receptors can lead to other changes in cells and tissues, not just endocrine disruptions [19].
It is now widely accepted that BPA not only has the effectiveness of E2 but is also potent in relation to several of its effects, that is, it has a potent estrogenic effect [8]. In addition, it can act as an anti-oestrogen, blocking the oestrogen response by competing with endogenous E2. It can also bind directly to androgen receptors (AR), possibly being anti-androgenic, blocking endogenous androgen action [22].
Endogenous oestrogens have various effects at the biological level, from every organism to the systems of organs, cells, and gene expression [8]. They play a key role in the testicle since their biosynthesis occurs in the testicular cells. The absence of ERs causes adverse effects on spermatogenesis and steroid synthesis. Physiological levels of E2 are essential for normal spermatogenesis, and when altered, it can lead to the occurrence of pathologies. For example, in infertility, there is an excess of E2 together with a decrease in testosterone [23]. In addition to its estrogenic activity, there is some evidence that BPA binds to the thyroid hormone receptor, acting as an antagonist and preventing the binding of T3 [8]. The affinity for the thyroid receptor is lower than the affinity for the oestrogen receptor, suggesting that elevated BPA levels would be needed to antagonise the hormonal action of the thyroid [7].
Regarding the male reproductive system, this substance perturbates the hypothalamic–pituitary–testicle axis and modulates gene expression and enzymatic activity of testicular steroidogenesis (Figure 2). It is also associated with a decrease in the activity of the antioxidant system, resulting in an increase in oxidative stress, the most common cause of sperm damage [24]. It exerts multidirectional effects as it interacts with several receptors, generates reactive oxygen species, alters cell signalling, causes mutagenic changes, and inhibits DNA methylation [10].
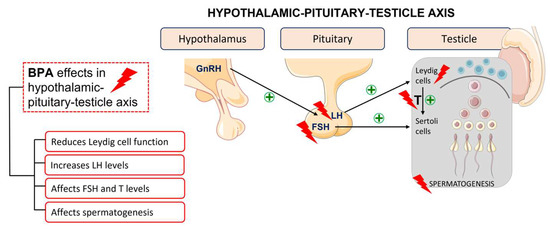
Figure 2. BPA effects in the hypothalamic–pituitary–testicle axis.
Although the mechanisms of action are not yet fully understood, BPA also interferes with other functions of the body, including the development of the central nervous system, the endocrine action of the pancreas, the immune system, and the function of sex hormones, insulin, leptin, and adiponectin. These BPA effects may also be responsible for the development of several types of cancer, such as breast, prostate, pancreas, pituitary, cerebellar cortex, and heart cancer [10][22].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241512417
References
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405.
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258.
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environ. Health Perspect. 1996, 104 (Suppl. S4), 715–740.
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342.
- Vandenberg, L.N.; Hunt, P.A.; Gore, A.C. Endocrine disruptors and the future of toxicology testing—Lessons from CLARITY-BPA. Nat. Rev. Endocrinol. 2019, 15, 366–374.
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210.
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34.
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95.
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575.
- Michalowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758.
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89.
- Sharma, B.M.; Bharat, G.K.; Chakraborty, P.; Martinik, J.; Audy, O.; Kukucka, P.; Pribylova, P.; Kukreti, P.K.; Sharma, A.; Kalina, J.; et al. A comprehensive assessment of endocrine-disrupting chemicals in an Indian food basket: Levels, dietary intakes, and comparison with European data. Environ. Pollut. 2021, 288, 117750.
- Bolognesi, C.; Castle, L.; Cravedi, J.-P.; Engel, K.-H.; Fowler, P.A.F.; Franz, R.; Grob, K.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978.
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambre, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e06857.
- Cannarella, R.; Gul, M.; Rambhatla, A.; Agarwal, A. Temporal decline of sperm concentration: Role of endocrine disruptors. Endocrine 2023, 79, 1–16.
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34.
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177.
- Dekant, W.; Volkel, W. Human exposure to bisphenol A by biomonitoring: Methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 2008, 228, 114–134.
- Santiago, J.; Silva, J.V.; Santos, M.A.S.; Fardilha, M. Fighting Bisphenol A-Induced Male Infertility: The Power of Antioxidants. Antioxidants 2021, 10, 289.
- Lorigo, M.; Cairrao, E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Asp. Med. 2022, 87, 101054.
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155.
- Vitku, J.; Sosvorova, L.; Chlupacova, T.; Hampl, R.; Hill, M.; Sobotka, V.; Heracek, J.; Bicikova, M.; Starka, L. Differences in bisphenol A and estrogen levels in the plasma and seminal plasma of men with different degrees of infertility. Physiol. Res. 2015, 64, S303–S311.
- Barbagallo, F.; Condorelli, R.A.; Mongioi, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Effects of Bisphenols on Testicular Steroidogenesis. Front. Endocrinol. 2020, 11, 373.
This entry is offline, you can click here to edit this entry!
