Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Genome editing has emerged as a powerful tool for accelerating crop improvement in horticultural crops by enabling precise modifications to their genetic makeup.
- CRISPR-Cas9
- crop improvement
- genome editing
- horticultural crops
1. Introduction to Genome Editing in Agriculture
Genome editing is a powerful biotechnological tool with transformative potential for horticultural crops. It involves the precise modification of plant DNA to enhance desired traits, increase crop yield, and confer resistance to pests, diseases, and environmental stresses. Molecular tools like CRISPR-Cas9, TALENs, and ZFNs act as molecular scissors, allowing for the targeted alteration of specific DNA sequences with unprecedented accuracy. This technology allows scientists to introduce or enhance beneficial traits in crops, including disease resistance, improved nutrition, and drought tolerance.
Horticulture is a critical component of global food production and human well-being as it involves the cultivation and management of plants for food, aesthetics, and medicinal purposes [1]. Horticultural crops, such as fruits, vegetables, ornamental plants, and medicinal herbs, not only contribute to the nutritional needs of populations worldwide but also enhance the visual appeal of our surroundings. However, the productivity and quality of horticultural crops face significant constraints due to challenges such as biotic and abiotic stresses, limited genetic variation, and increasing demands for improved traits [2].
Plant breeders have employed various techniques, such as hybridization, selection, and genetic manipulation, to enhance horticultural crops [3]. These approaches have contributed to improved yield, disease resistance, and other desirable traits [4]. However, traditional breeding methods have limitations, including long breeding cycles, limited genetic variation, and complex genetic architectures [5]. Genome editing has emerged as a transformative technology with the potential to revolutionize crop improvement, including horticultural crops [6]. By enabling precise modifications in an organism’s DNA, genome editing offers an efficient method to manipulate specific genes and traits [7]. This technology holds immense promise for expediting crop improvement, overcoming genetic barriers, and addressing specific challenges in horticultural crops.
The CRISPR-Cas9 system is widely used as a genome editing tool, utilizing guide RNA to direct the Cas9 enzyme for precise DNA cleavage and modifications [8]. TALENs and ZFNs are alternative genome editing tools also utilized in horticultural crop research [9]. These tools enable the precise editing of plant genomes by targeting specific genes associated with desired traits. CRISPR-Cas9 has proven effective in improving important traits in various horticultural crops through targeted modifications [10]. By designing specific gRNAs, researchers can direct the Cas9 enzyme to target genes associated with traits of interest, including disease resistance, abiotic stress tolerance, nutritional content, and yield-related characteristics [11]. The precise nature of CRISPR-Cas9 allows for the introduction of beneficial mutations or targeted gene knockouts, simulating natural genetic variations and accelerating the breeding process [12].
TALENs and ZFNs, along with CRISPR-Cas9, have been employed in horticultural crop research as genome editing tools (Figure 1). TALENs and ZFNs utilize engineered DNA-binding proteins that can be customized to target specific genomic sequences [13]. Similar to CRISPR-Cas9, these tools induce targeted DNA cleavage and subsequent modifications at the desired genomic sites. TALENs employ DNA-binding domains derived from transcription activator-like effectors (TALEs), which are naturally occurring proteins in plant pathogenic bacteria [14]. Engineered TALE domains are utilized to bind specific DNA sequences and are fused with a nuclease domain to induce DNA cleavage [15]. In contrast, ZFNs are hybrid proteins that combine engineered zinc finger DNA-binding domains with the FokI nuclease domain derived from the FokI restriction enzyme [16]. The zinc finger domains are designed to recognize targeted DNA sequences, and the FokI domain cleaves the DNA at the desired site [17].
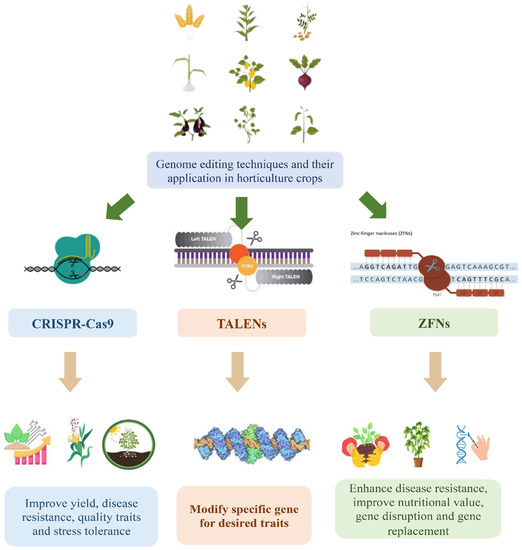
Figure 1. Genome editing in horticulture crops.
These genome editing tools offer researchers the ability to precisely modify genes associated with desired traits in horticultural crops, facilitating the development of improved varieties [18]. By utilizing these tools, scientists can expedite the enhancement of traits such as disease resistance, abiotic stress tolerance, nutritional content, and yield potential. In this review, we examine the applications, methodologies, and potential impacts of genome editing tools, including CRISPR-Cas9, TALENs, and ZFNs, in horticultural crops
.
2. CRISPR-Cas9 in Horticultural Crops
The CRISPR-Cas9 system has gained prominence for its user-friendly nature, efficiency, and adaptability in genetic manipulation across organisms, including horticultural crops [21]. Based on bacteria’s defense mechanism against viral infections, CRISPR-Cas9 is a powerful tool [22] that enables precise genome editing in plants. This technology empowers researchers to target specific genes associated with desirable traits and introduce modifications to enhance agricultural characteristics [23]. This level of precision allows for the introduction of advantageous mutations, gene disruption, and the substitution of specific DNA sequences, leading to desired alterations in traits such as disease resistance, abiotic stress tolerance, nutritional composition, and yield-related attributes [24].
Table 2 presents examples of horticultural crops that have undergone genome editing using the CRISPR-Cas9 technique, along with the specific genes targeted and the resulting modified genetic traits. These modifications have led to significant improvements in fruit ripening, disease resistance, flowering time, tuberization, grain quality, and other desirable characteristics in the respective crops.
Table 2. Horticultural crops subjected to genome editing techniques, modified genetic traits in various plant species.
| Crops | Modified Gene(s) | Trait/Function | Reference |
|---|---|---|---|
| Tomato | E8, Phytoene desaturase (PDS), SlDELLA | Enhanced fruit ripening, delayed fruit senescence, reduced plant height | [25] |
| Potato | StCDF1 | Increased tuberization and yield | [26] |
| Wheat | TaGW2, Puroindoline genes | Enhanced thousand grain weight, improved grain quality | [27] |
| Citrus | CsPDS | Improved disease resistance, reduced ethylene production | [28] |
| Strawberry | FaTM6 | Petal and stamen development | [29] |
| Grape | VvWRKY52, VvWRKY2 | Enhanced disease resistance, improved abiotic stress tolerance | [30] |
| Brassica oleracea | XccR5-89.2 | Improved resistance to blackleg disease | [31] |
| Mushroom (Agaricus bisporus) | Polyphenol oxidase (PPO) genes | Reduced browning and improved shelf life | [32] |
| Banana | MaACO1 | Promotes the shelf life of banana | [33] |
| Carrot | DcCCD4 | Different colored taproots in carrots | [34] |
| Strawberry | FaGAST1 | Increased fruit size | [35] |
| Cucumis melo | CmACO1 | Extends the shelf-life | [36] |
| Capsicum annuum | CaERF28 | Anthracnose resistance | [37] |
| Rose | RhEIN2 | Ethylene insensitivity in rose | [38] |
| Melon | eIF4E | Virus resistance and male sterility | [39] |
| Tomato | SlMAPK3 | Reduced drought tolerance | [40] |
| Brassica napus | FAD2 | Catalyzes the desaturation of oleic acid | [41] |
| Kiwifruit | AcBFT | Reduce plant dormancy | [42] |
| Tomato | SlMYC2 | Fruit Resistance to Botrytis cinerea | [43] |
| Soybean | GmFATB1 | Reduce saturated fatty acids | [44] |
| Kiwi fruit | AcCBF3 | Dwarf plants and enhanced freezing tolerance | [45] |
| Sweet Potato | IbGBSSI and IbSBEII | Improvement of starch quality | [46] |
| Papaya | phytoene desaturase (CpPDS) | Inducing a visually scorable albino phenotype | [47] |
| Eggplant | SmelPPO4, SmelPPO5, and SmelPPO6 | Reduces fruit flesh browning | [48] |
| Cassava | eIF4E | Reduces cassava brown streak disease symptom | [49] |
The limited genetic diversity in horticultural crops poses challenges for developing improved varieties with enhanced traits [50]. CRISPR-Cas9 facilitates the accurate introduction of genetic variations, replicating the genetic diversity observed in wild relatives or closely related species [51]. Targeted modifications of specific genes or regulatory elements unlock untapped genetic potential and expand the available variation for crop enhancement [52], fostering the development of resilient, productive, and nutritionally valuable horticultural crops.
3. TALENs (Transcription Activator-like Effector Nucleases)
TALENs, along with CRISPR-Cas9, are widely employed as genome editing tools for precise gene modifications in horticultural crops [114]. TALENs are engineered nucleases capable of inducing double-strand breaks (DSBs) at specific DNA sequences, enabling targeted gene editing [128]. Comprising a customizable DNA-binding domain derived from transcription activator-like effectors (TALEs) and a nuclease domain typically derived from the FokI endonuclease, TALENs offer a dual-component design [129].
The DNA-binding domain of TALENs is constructed using multiple repeats of TALEs, each recognizing a specific nucleotide in the target DNA sequence [130]. These TALEs consist of repeat units typically containing 33–35 amino acids in a central repeat region [131]. The specificity of TALENs is achieved through customizable repeat variable di-residues (RVDs) within each repeat unit, where different RVDs recognize different nucleotides, enabling the design of highly specific TALENs [132].
The nuclease domain of TALENs is derived from the FokI endonuclease, which requires dimerization for its DNA cleavage activity [133]. TALENs are designed as pairs, with each TALEN targeting one DNA strand [134]. Upon binding to their target sites, the FokI nuclease domains of the TALENs dimerize, forming a functional nuclease complex that induces double-strand breaks (DSBs) at the target site [135].
4. ZFNs (Zinc Finger Nucleases)
ZFNs, an engineered nuclease class, have been utilized for genome editing in horticultural crops [159]. Comprising two main components, ZFPs and a nuclease domain from FokI endonuclease, ZFNs exhibit sequence-specific DNA recognition [160,161]. Each zinc finger module, approximately 30 amino acids in length, targets three DNA bases, and multiple modules enable precise targeting of specific DNA sequences [162,163]. ZFNs are employed in pairs, with each ZFN targeting one DNA strand [164]. The binding of the ZFNs to their target sites leads to FokI nuclease domain dimerization, generating a functional nuclease complex that induces double-strand breaks (DSBs) at the target site [135].
5. Regulatory and Ethical Considerations
Genome editing has emerged as a promising approach for sustainable agriculture and crop enhancement, aiming to create transgene-free plants [186]. However, to ensure responsible and safe utilization of this technology, it is imperative to address key regulatory and ethical aspects. As genome editing advances, a thorough understanding of international regulations, regional policies, and ethical implications becomes essential in promoting its widespread adoption for crop improvement in agriculture.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9080884
This entry is offline, you can click here to edit this entry!
