Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Prenatal genetic screening tests are ”non-invasive” methods that include the maternal serum screening of markers related to aneuploidy with or without nuchal translucency ultrasonography, and cell-free DNA screening, known as non-invasive prenatal testing (NIPT). These tests are performed by drawing maternal peripheral blood sampling; therefore, there is no risk of procedure-related pregnancy complications.
- NIPT
- prenatal genetic screening
- diagnostic testing
- non-invasive
- false-positive
- fetal fraction
1. Introduction
One of the latest phenomena in current obstetrics is advancing maternal age. Recent data from the Centers for Disease Control and Prevention (CDC) emphasized the rising trend in the average age of pregnant women in the United States in which almost 19% of all pregnancies were in maternal age of 35 years and older [1]. This trend is observed globally, especially with women’s age at their first pregnancy continuously advancing at a fast pace. In particular, South Korea has drawn attention to its fast change in the average age of first childbirth increasing from 26 in 1993 to 32 in 2020 [2]. Therefore, recent obstetrical consensus had repeatedly addressed major complications in pregnancies with advancing maternal age, including increased risk of fetal aneuploidy which is mentioned with the greatest emphasis [3][4]. The Obstetrical Care Consensus statement in 2022 for “pregnancy at age 35 years or older” by the American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine states that prenatal genetic screening and diagnostic testing options should be offered with detailed counseling based on each patient’s individual risk [5]. Therefore, prenatal genetic counseling has become one of the most imperative tasks for a clinician in current obstetrics.
At present, prenatal genetic screening and diagnostic testing are two separate categories that can be characterized by their invasiveness. Since non-invasive prenatal testing (NIPT) is only for screening purpose, when the results show a high risk of aneuploidy, confirmative diagnostic testing is mandatory. The diagnostic tests include chorionic villus sampling (CVS) and amniocentesis which are ”invasive”. Since the tests require a needle puncturing through the uterine wall to the actual placenta or amniotic fluid, the risks of complication exist with a fetal loss rate of 0.7% and 0.6% for CVS and amniocentesis, respectively [6].
Therefore, pregnant women tend to prefer non-invasive prenatal screening to invasive diagnostic testing which has led to the rapid spread of NIPT around the world ever since its commercial introduction in 2011 [7]. NIPT is now available in more than 60 countries and the annual growth rate of NIPT has been estimated to be about 10.9% to 17.15% [7]. The estimated global market value for NIPT ranged from USD 2.8 to 3.9 billion in 2019–2020 with the United States accounting for the biggest market share [8]. However, various limitations of NIPT have been continuously raised which have restricted its use as a prenatal genetic test for screening purposes only. Thus, NIPT is often referred to as “NIPS” (non-invasive prenatal screening) since pregnant women can often be confused by the concept of ”screening” and ”diagnostic” and the name of NIPT itself can be misleading [9].
In many countries, NIPT is used as a secondary test for women identified as high-risk after the first trimester combined screening, and in others, it is used as a primary screening test. As compared to the prior screening methods for aneuploidy involving sequential maternal serum and/or ultrasound screening, which exhibits trisomy 21 detection rates of 81–96% with a false-positive rate fixed at 5%, NIPT demonstrates higher detection rates for trisomy 21 (99%), and also for trisomy 18 (98%) and trisomy 13 (99%) with much lower false-positive rates of 1–2% [10][11][12][13]. The American College of Medical Genetics now recommends NIPT as a primary screening test for sex chromosome abnormality as well as for fetal trisomies 21, 18, 13 [14].
2. Fetal Fraction (FF)
However, NIPT exhibits quite a few limitations to consider. First of all, maternal blood contains only a small fraction of fetal cells (1 fetal cell per 106–107 maternal cells) [15]. For the test to be adequately analyzed, a fetal fraction (FF) of cfDNA, which originates from placental cytotrophoblasts in a maternal blood sample, must exceed at least 2–4% of the total cfDNA in the plasma and this can be challenging under various circumstances [16][17]. Numerous factors can result in a low FF either by increasing maternal cfDNA amounts or decreasing fetal (placental) cfDNA concentrations. The most important maternal factor is the maternal body weight; an increased maternal body mass index (BMI) is related to inflammation and necrosis of adipocytes which in turn increases the maternal-derived cfDNA concentrations [18]. Therefore, according to previous study reports, the no-call (unreportable results) rate for NIPT ranges from 5.4% to 70.1% for women with a BMI higher than 40, compared to 0% to 4.2% for women with a BMI between 18.5 and 24.9 [19]. Other maternal factors that can alter the cfDNA concentration include pre-existing maternal diseases, drug use, and assisted reproductive pregnancy [20][21][22].
During pregnancy, women with autoimmune disease may experience a low level of cffDNA as an inflammatory response may cause a rise in maternal cfDNA in the bloodstream. This ultimately leads to a decline in the proportion of cfDNA derived from the fetus. Systemic lupus erythematosus (SLE) patients may exhibit abnormal DNA methylation in their T cells, which, along with T cell apoptosis, can further contribute to an increase in hypomethylated cfDNA in the blood with shorter DNA fragment lengths. Consequently, NIPT analyses using next-generation sequencing may reveal a different pattern in SLE patients compared to healthy patients [23].
Suzumori et al. showed that the concentration of cffDNA decreases in cases of trisomy 13 or 18. The smaller placental size and intrauterine fetal growth retardation observed with trisomy 13 and 18 might contribute to a lower FF [24]. Taglauer et al. reported that fetuses with trisomy 21 have an increased FF when compared to euploid fetuses [25]. This may be reflective of a better test performance for trisomy 21 as compared to trisomy 13 or 18 [26]. The most important fetal factor is the gestational age at the time of blood sampling since the FF increases as the fetus and the placenta grow throughout pregnancy [27]. As of today’s technology, the gestational age should be at least 9–10 weeks for NIPT to be reliably tested in a singleton pregnancy [28]. Hou et al. reported that the percentage of the fetal fraction significantly increased with the increasing gestational age. On the other hand, a decrease in the fetal fraction was observed with an increasing maternal BMI [29].
3. Chromosomal Mosaicism
False-positive test results for NIPT may arise due to confined placental mosaicism (CPM) [30]. Since the primary source of “fetal” cfDNA in maternal circulation is placental cells (syncytiotrophoblasts), the cfDNA test is expected to provide results relevant to the placenta, which may be discordant with the actual fetal tissue. Previous CVS cases have shown that discordance may occur in 1–2% of pregnancies [30][31][32][33][34], and is more likely in cases with monosomy X and trisomy 13 than those with trisomy 21 or 18 [35]. True fetal mosaicism (TFM) can also result in false-negative cfDNA results (where the fetus is affected but cfDNA testing indicates no chromosomal abnormality). Although it is quite rare, false negatives in cfDNA results have been reported in Japanese data by a percentage of 0.01% [36]. In these cases, the fetus was chromosomally abnormal but cells with normal karyotype chromosomes existed on the villi [37]. Since the results of NIPT are determined by the relative proportions of cells with normal and abnormal karyotypes within the villi, mosaicism in which a high proportion of cells with normal karyotypes exist may produce a negative result. Since the NIPT result is negative, a definitive diagnostic test is not performed despite the fetus being chromosomally abnormal. In these cases, the cfDNA test results are considered analytically correct (i.e., detecting those placental cells of the mosaicism which are euploid) but clinically incorrect (i.e., the fetus itself is aneuploid) [38]. This kind of situation can occur for trisomy 13 and 18, but not trisomy 21 [39] (Figure 1).
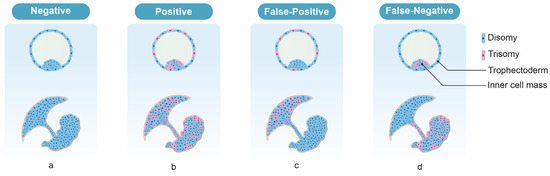
Figure 1. The expected NIPT results according to the types of mosaicism. (a) Euploid fetus and placenta, (b) generalized mosaicism, (c) CPM, (d) TFM, in which trisomy cells are absent in the cytotrophoblast but present in the villus mesenchyme and the fetus.
4. Maternal Malignancies
Chromosomal abnormalities are often present in malignant tumors. If a pregnant woman has a malignant tumor, the NIPT result may be a false positive or may be non-reportable. The risk of confirmed malignancy is significantly higher especially when multiple chromosomal abnormalities are detected by NIPT [38][40]. Catharina et al. reported that a low percentage (0.02%) of NIPT results were assessed as indicative of a maternal malignancy in 231,896 pregnant women [40]. Although relatively rare, malignant tumors occur in about 1 in 1000 pregnant women [41] and account for approximately 15% of false-positive NIPT results [30]. An amniotic fluid examination is performed in NIPT-positive pregnant women spanning multiple chromosomes and if there is no chromosomal abnormality in the fetus, it is necessary to be cautious about the combination of malignant tumors [38]. False positives due to benign tumors such as fibroids have also been reported [42].
5. Vanishing Twins (VT)
A vanishing twin (VT) is a spontaneous reduction of an embryo and/or gestational sac following documented fetal cardiac activity in both fetuses of a twin gestation during the first trimester [43]. Since chromosomal abnormalities are one of the major causes of miscarriages, trisomies could be the cause of VT, leading to a potentially high number of false-positive results. To avoid inaccurate results, recent guidelines from the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine suggest diagnostic testing in multifetal pregnancies if a vanishing twin is identified, instead of relying on serum-based aneuploidy screening or cfDNA [13]. However, other systematic reviews have shown that NIPT can successfully detect common autosomal aneuploidies in pregnancies affected by VT, although with a higher false-positive rate [44].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13152532
References
- Osterman, M.; Hamilton, B.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020. Natl. Vital. Stat. Rep. 2021, 70, 17.
- OECD. OECD Economic Surveys: Korea 2022; OECD Publishing: Paris, France, 2022.
- Frick, A.P. Advanced maternal age and adverse pregnancy outcomes. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 92–100.
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Womens Health 2021, 13, 751–759.
- Gantt, A.; Metz, T.D.; Kuller, J.A.; Louis, J.M.; Cahill, A.G.; Turrentine, M.A. Obstetric Care Consensus #11, Pregnancy at age 35 years or older. Am. J. Obstet. Gynecol. 2022, 228, B25–B40.
- Akolekar, R.; Beta, J.; Picciarelli, G.; Ogilvie, C.; D’Antonio, F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review and meta-analysis. Ultrasound Obs. Gynecol 2015, 45, 16–26.
- Ravitsky, V.; Roy, M.-C.; Haidar, H.; Henneman, L.; Marshall, J.; Newson, A.J.; Ngan, O.M.Y.; Nov-Klaiman, T. The Emergence and Global Spread of Noninvasive Prenatal Testing. Annu. Rev. Genom. Hum. Genet. 2021, 22, 309–338.
- Allyse, M.; Minear, M.A.; Berson, E.; Sridhar, S.; Rote, M.; Hung, A.; Chandrasekharan, S. Non-invasive prenatal testing: A review of international implementation and challenges. Int. J. Womens Health 2015, 7, 113–126.
- Quaresima, P.; Visconti, F.; Greco, E.; Venturella, R.; Di Carlo, C. Prenatal tests for chromosomal abnormalities detection (PTCAD): Pregnant women’s knowledge in an Italian Population. Arch Gynecol Obstet. 2021, 303, 1185–1190.
- Malone, F.D.; Canick, J.A.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Bukowski, R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N. Engl. J. Med. 2005, 353, 2001–2011.
- Bianchi, D.W.; Platt, L.D.; Goldberg, J.D.; Abuhamad, A.Z.; Sehnert, A.J.; Rava, R.P. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obs. Gynecol 2012, 119, 890–901.
- Zimmermann, B.; Hill, M.; Gemelos, G.; Demko, Z.; Banjevic, M.; Baner, J.; Ryan, A.; Sigurjonsson, S.; Chopra, N.; Dodd, M.; et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat. Diagn. 2012, 32, 1233–1241.
- Rose, N.C.; Kaimal, A.J.; Dugoff, L.; Norton, M.E.; American College of Obstetricians and Gynecologists. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet. Gynecol. 2020, 136, e48–e69.
- Dungan, J.S.; Klugman, S.; Darilek, S.; Malinowski, J.; Akkari, Y.M.N.; Monaghan, K.G.; Erwin, A.; Best, R.G. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2023, 25, 100336.
- Steele, C.D.; Wapner, R.J.; Smith, J.B.; Haynes, M.K.; Jackson, L.G. Prenatal diagnosis using fetal cells isolated from maternal peripheral blood: A review. Clin Obs. Gynecol 1996, 39, 801–813.
- Wong, F.C.; Lo, Y.M. Prenatal Diagnosis Innovation: Genome Sequencing of Maternal Plasma. Annu. Rev. Med. 2016, 67, 419–432.
- Fiorentino, F.; Bono, S.; Pizzuti, F.; Mariano, M.; Polverari, A.; Duca, S.; Sessa, M.; Baldi, M.; Diano, L.; Spinella, F. The importance of determining the limit of detection of non-invasive prenatal testing methods. Prenat. Diagn. 2016, 36, 304–311.
- Vora, N.L.; Johnson, K.L.; Basu, S.; Catalano, P.M.; Hauguel-De Mouzon, S.; Bianchi, D.W. A multifactorial relationship exists between total circulating cell-free DNA levels and maternal BMI. Prenat. Diagn. 2012, 32, 912–914.
- Juul, L.A.; Hartwig, T.S.; Ambye, L.; Sørensen, S.; Jørgensen, F.S. Noninvasive prenatal testing and maternal obesity: A review. Acta Obstet. Gynecol. Scand. 2020, 99, 744–750.
- Putra, M.; Idler, J.; Patek, K.; Contos, G.; Walker, C.; Olson, D.; Hicks, M.A.; Chaperon, J.; Korzeniewski, S.J.; Patwardhan, S.C.; et al. The association of HBB-related significant hemoglobinopathies and low fetal fraction on noninvasive prenatal screening for fetal aneuploidy. J. Matern. Fetal. Neonatal. Med. 2021, 34, 3657–3661.
- Kuhlmann-Capek, M.; Chiossi, G.; Singh, P.; Monsivais, L.; Lozovyy, V.; Gallagher, L.; Kirsch, N.; Florence, E.; Petruzzi, V.; Chang, J.; et al. Effects of medication intake in early pregnancy on the fetal fraction of cell-free DNA testing. Prenat. Diagn. 2019, 39, 361–368.
- Deng, C.; Liu, S. Factors Affecting the Fetal Fraction in Noninvasive Prenatal Screening: A Review. Front. Pediatr. 2022, 10, 812781.
- Chan, R.W.; Jiang, P.; Peng, X.; Tam, L.S.; Liao, G.J.; Li, E.K.; Wong, P.C.; Sun, H.; Chan, K.C.; Chiu, R.W.; et al. Plasma DNA aberrations in systemic lupus erythematosus revealed by genomic and methylomic sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, E5302–E5311.
- Suzumori, N.; Ebara, T.; Yamada, T.; Samura, O.; Yotsumoto, J.; Nishiyama, M.; Miura, K.; Sawai, H.; Murotsuki, J.; Kitagawa, M.; et al. Fetal cell-free DNA fraction in maternal plasma is affected by fetal trisomy. J. Hum. Genet. 2016, 61, 647–652.
- Taglauer, E.S.; Wilkins-Haug, L.; Bianchi, D.W. Review: Cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta 2014, 35, S64–S68.
- Bianchi, D.W.; Wilkins-Haug, L. Integration of noninvasive DNA testing for aneuploidy into prenatal care: What has happened since the rubber met the road? Clin. Chem. 2014, 60, 78–87.
- Kinnings, S.L.; Geis, J.A.; Almasri, E.; Wang, H.; Guan, X.; McCullough, R.M.; Bombard, A.T.; Saldivar, J.S.; Oeth, P.; Deciu, C. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat. Diagn. 2015, 35, 816–822.
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314.
- Hou, Y.; Yang, J.; Qi, Y.; Guo, F.; Peng, H.; Wang, D.; Wang, Y.; Luo, X.; Li, Y.; Yin, A. Factors affecting cell-free DNA fetal fraction: Statistical analysis of 13,661 maternal plasmas for non-invasive prenatal screening. Hum Genom. 2019, 13, 62.
- Hartwig, T.S.; Ambye, L.; Sørensen, S.; Jørgensen, F.S. Discordant non-invasive prenatal testing (NIPT)—A systematic review. Prenat. Diagn. 2017, 37, 527–539.
- Malvestiti, F.; Agrati, C.; Grimi, B.; Pompilii, E.; Izzi, C.; Martinoni, L.; Gaetani, E.; Liuti, M.R.; Trotta, A.; Maggi, F.; et al. Interpreting mosaicism in chorionic villi: Results of a monocentric series of 1001 mosaics in chorionic villi with follow-up amniocentesis. Prenat. Diagn. 2015, 35, 1117–1127.
- Kalousek, D.K.; Vekemans, M. Confined placental mosaicism. J. Med. Genet. 1996, 33, 529–533.
- Schreck, R.R.; Falik-Rorenstein, Z.; Hirata, G. Chromosomal Mosaicism in Chorionic Villus Sampling. Clin. Perinatol. 1990, 17, 867–888.
- Kalousek, D.K.; Howard-Peebles, P.N.; Olson, S.B.; Barrett, I.J.; Dorfmann, A.; Black, S.H.; Schulman, J.D.; Wilson, R.D. Confirmation of CVS mosaicism in term placentae and high frequency of intrauterine growth retardation association with confined placental mosaicism. Prenat. Diagn. 1991, 11, 743–750.
- Grati, F.R.; Bajaj, K.; Malvestiti, F.; Agrati, C.; Grimi, B.; Malvestiti, B.; Pompilii, E.; Maggi, F.; Gross, S.; Simoni, G.; et al. The type of feto-placental aneuploidy detected by cfDNA testing may influence the choice of confirmatory diagnostic procedure. Prenat. Diagn. 2015, 35, 994–998.
- Samura, O.; Sekizawa, A.; Suzumori, N.; Sasaki, A.; Wada, S.; Hamanoue, H.; Hirahara, F.; Sawai, H.; Nakamura, H.; Yamada, T.; et al. Current status of non-invasive prenatal testing in Japan. J. Obstet. Gynaecol. Res. 2017, 43, 1245–1255.
- Grati, F.R.; Malvestiti, F.; Ferreira, J.C.P.B.; Bajaj, K.; Gaetani, E.; Agrati, C.; Grimi, B.; Dulcetti, F.; Ruggeri, A.M.; De Toffol, S.; et al. Fetoplacental mosaicism: Potential implications for false-positive and false-negative noninvasive prenatal screening results. Genet. Med. 2014, 16, 620–624.
- Samura, O.; Okamoto, A. Causes of aberrant non-invasive prenatal testing for aneuploidy: A systematic review. Taiwan J Obs. Gynecol 2020, 59, 16–20.
- Kalousek, D.K.; Barrett, I.J.; McGillivray, B.C. Placental mosaicism and intrauterine survival of trisomies 13 and 18. Am. J. Hum. Genet 1989, 44, 338–343.
- Heesterbeek, C.J.; Aukema, S.M.; Galjaard, R.H.; Boon, E.M.J.; Srebniak, M.I.; Bouman, K.; Faas, B.H.W.; Govaerts, L.C.P.; Hoffer, M.J.V.; den Hollander, N.S.; et al. Noninvasive Prenatal Test Results Indicative of Maternal Malignancies: A Nationwide Genetic and Clinical Follow-Up Study. J. Clin. Oncol. 2022, 40, 2426–2435.
- Pavlidis, N.A. Coexistence of Pregnancy and Malignancy. Oncol. 2002, 7, 279–287.
- Dharajiya, N.G.; Namba, A.; Horiuchi, I.; Miyai, S.; Farkas, D.H.; Almasri, E.; Saldivar, J.-S.; Takagi, K.; Kamei, Y. Uterine leiomyoma confounding a noninvasive prenatal test result. Prenat. Diagn. 2015, 35, 990–993.
- Levi, S. Ultrasonic assessment of the high rate of human multiple pregnancy in the first trimester. J. Clin. Ultrasound 1976, 4, 3–5.
- van Eekhout, J.C.A.; Bekker, M.N.; Bax, C.J.; Galjaard, R.-J.H. Non-invasive prenatal testing (NIPT) in twin pregnancies affected by early single fetal demise: A systematic review of NIPT and vanishing twins. Prenat. Diagn. 2023, 42, 829–837.
This entry is offline, you can click here to edit this entry!
