Bone marrow is known as the site of hematopoiesis. What is not being described in textbooks of immunology is the fact that bone marrow is not only a generative, but also an antigen-responsive, immune organ. It is also a major storage site for antigen-specific memory B and T cells.
- immunological synapse
- antigen-presenting cell
- mesenchymal stem cell
- memory T cell
- regenerative medicine
- T regulatory cell
- bone marrow stromal niche
1. Introduction
2. Bone Marrow: A Hematopoietic and Antigen-Responsive Lymphatic Organ
2.1. BM: A Central Organ Protected by Bone
2.2. BM: A Central Hematopoietic Organ
2.3. BM: A Central Antigen Responsive Lymphatic Organ
2.4. Active Control of Proliferating Tumor Cells by CD8+ Memory T Cells Leading to Tumor Dormancy in BM
3. Comparison between BM and Blood
3.1. Comparison of DC Generation from Mononuclear Cells and Their Function
3.2. Enrichment of Memory T Cells in BM of Breast Cancer Patients
3.3. Generation of Tumor-Specific CTL from BM, but Not PBL, of Breast Cancer Patients
3.4. Superior Therapeutic Efficiency In Vivo of Reactivated MTCs from BM in Comparison to Blood of Breast Cancer Patients
4. BM Storage Capacity for Memory B and Memory T Cells
4.1. Survival Niches for Memory B and Memory Plasma Cells in BM Parenchyma
4.2. Memory T Cells
4.3. Survival Niches for Memory T Cells in BM Parenchyma
4.4. Tissue-Resident Memory T Cells in BM Parenchyma
4.5. Stem-like Memory T Cells in BM Parenchyma
4.6. Enrichment of Virus-Specific MTCs in Human BM Parenchyma
4.7. Cognate Re-Activation of TA-Specific BM MTCs Ex Vivo and In Situ
4.8. Hypotheses for the Maintenance of Long-Term Memory in the BM
-
Quiescence: Following the successful resolution of an immune reaction, antibody-secreting memory plasma cells and memory B and T cells persist as quiescent cells (non-proliferative, non-migratory) in dedicated survival niches organized by BM stromal cells. The immune memory cells dock individually onto dedicated stromal cells, which control their maintenance. The number of available dedicated stromal cells defines the size of the memory compartment [46].
-
Cognate re-activation of BM memory cells. Upon re-encounter with the antigen, which enters directly via the blood into the vascularized BM or is transported there by APCs, antigen-specific memory B and MTCs are re-activated. MTCs proliferate locally, form immune clusters, and provide local protection. Others exit the BM and contribute to secondary immune reactions in the periphery. BM clusters in the parenchyma can develop into large follicles. These include memory B and memory plasma cells in addition to CD4+ MTCs, suggesting T–B cell interaction [47]. Once a BCR binds its T cell-dependent antigen, the antigen is taken up into the B cell through receptor-mediated endocytosis. This is then degraded and presented to T cells as a p-MHC II complex at the cell membrane. Memory B cells in immune follicles might receive stimulatory signals from antigen-specific helper T cells upon T–B synapse formation. More than one antigenic determinant of a protein is required for such antigen-specific T-B cell interactions [48], one interacting with the BCR, the other with the TCR. Thus, activated memory B cells may directly differentiate into antibody-secreting cells in the BM, providing rapid enhancement of humoral immunity [46][47].
5. Bone Marrow Vaccination or Allogeneic BM Cell Injection: Novel Approaches to Enhance or Reduce Antigen-Specific Immunity
6. Interactions in BM between Three Types of Stem Cells and Immune Cells
6.1. Hematopoietic Stem Cells (HSCs) in Cross-Talk with T Cells and DCs
6.2. Extramedullary HSCs in Meninges of Adult Mice Providing Immune Surveillance of the CNS
6.3. BM Neural Crest-Derived Stem Cells Affecting B Cell Lymphopoiesis
6.4. Mesenchymal Stem Cells in Cross-Talk with T Cells
7. Effect of Dietary Restriction (DR) on the BM
7.1. Effect of DR on Monocytes from the BM
7.2. Effect of DR on Mucosal Immune Responses: Migration of Naïve B Cells from PPs to BM
7.3. Effects of DR on Memory T Cells: BM as a Refuge for Immune Memory
8. Blood-Borne Antigens, Circulating Cells, and Subcellular Particles
8.1. Self and Non-Self Antigens
8.2. Circulating Tumor Cells, Tumor-Associated Antigens, and Immunogenic Cell Death
8.3. Circulatory Antigen-Presenting DCs and Their Homing to BM
8.4. Circulatory Naïve T and Memory T Lymphocyte Subsets
8.5. CNS-Derived Antigens, CNS Immunosurveillance, and Cells Traveling through Cerebrospinal Fluid into Venous Blood
9. Neuro-Immune and Neuro-Osteogenic Links, Pathologies, and Interventions
9.1. Neuro-Immune Links
9.2. Pathologies and Interventions
- (i)
-
CNS lymphoma. In primary CNS lymphoma, attention has turned to the long-term outcomes of consolidation therapies, and recent studies have highlighted the excellent disease control afforded by high-dose chemotherapy and stem cell transplantation [78].
- (ii)
-
Malignant glioma (GBM). The glioma immune landscape has been described as a double-edged sword for treatment [79]. There are the effects of tumor cells on the tumor microenvironment, the immunosuppressive effects of myeloid immune cells, and the lymphocyte responses against the glioma cells [79]. Clinical and translational advances in malignant glioma immunotherapy have been summarized recently [80].
- (iii)
- (iv)
-
Chimeric antigen-receptor (CAR) T cells. Chimeric antigen receptor (CAR) T-cell therapy is a new and emerging cell therapy which has achieved remarkable success in the treatment of hematological malignancies [82]. The side effects include prolonged cytopenia (PC). Cytokine analysis after CAR T-cell infusion showed that CXCL12 and stem cell factor were significantly decreased in the BM of patients with PC, suggesting reduced niche cell function [83].
9.3. Neuro-Osteogenic Network
Skeletal tissue is highly innervated. The hallmarks of peripheral nerve function in bone regeneration were reviewed in [84]. The review summarizes the ways in which the peripheral nervous system (PNS) communicates with bone-lineage cells, the vasculature, and immune cells in the bone microenvironment [84]. It was concluded that the PNS regulates bone regeneration through neuropeptides or neurotransmitters and cells in the peripheral nerves [84].
10. Bone Marrow–Blood Interaction
10.1. BM Capacity for Cognate T Cell–APC Interactions
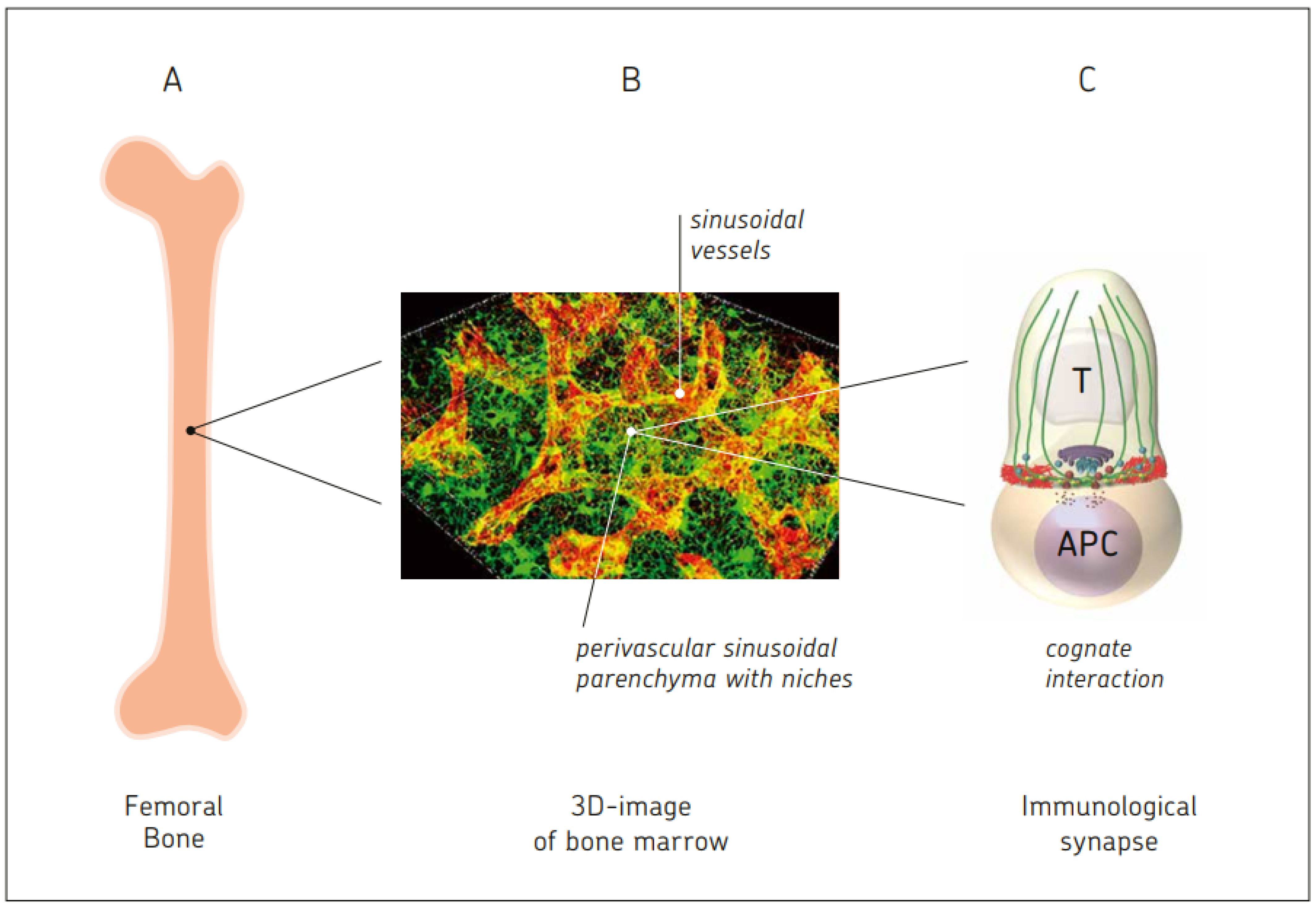
10.2. Effects of MSC-Derived Stromal Cells in the BM
- (i)
-
Stromal cell–immune cell contact-dependent PI3K and APRIL induces NF-kB signaling and prevents mitochondrial and ER stress of memory plasma cells [101];
- (ii)
-
Stromal cell CD80/CD86 expression provides CD28 stimulation in BM-resident plasma cells, leading to sustained antibody responses [102];
- (iii)
-
Stromal cells providing superior bio-availability of IL-15 cause upregulation of glucocortioid-induced TNF receptor (GITR) on CD8 MTCs [103];
- (iv)
-
Stromal-cell-derived Il-7 mediates homeostasis of naïve and memory CD8 T cells in vivo [104];
- (v)
-
Stromal-cell-expressed VCAM-1 holds immune cells in the niche and maintains plasma cell longevity [105].
10.3. Autonomous BM-Derived Adaptive Immune Response
11. Bone Marrow: T Regulatory Cells and Dendritic Cells Reacting to Cancer and Microbial Infection
11.1. Epigenetic Regulation
11.2. BM and Treg Cells
11.3. GvL without GvH
11.4. Treg Cells in BM of Ewing Sarcoma Patients
11.5. Tumor-Specific BM Treg Cells in Breast Cancer Patients
11.6. Effect of Microbial Infection and Inflammation on BM
11.6.1. Sepsis
11.6.2. Severe Malaria
11.6.3. Colitis
12. Tumor Dormancy in BM and Maintenance of Tumor-Specific MTCs
13. Bone Marrow Mesenchymal Stem Cells and Stromal Cells
This entry is adapted from the peer-reviewed paper 10.3390/immuno3030019
References
- Flajnik, M.F.; Kasahara, M. Origin and evolution of the adaptive immunity system: Genetic events and selective pressures. Nat. Rev. Genet. 2010, 11, 47–59.
- Pancer, Z.; Amemiya, C.T.; Ehrhardt, G.R.A.; Ceitlin, J.; Gartland, G.L.; Cooper, M.D. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature 2004, 430, 174–180.
- Estefa, J.; Tafforeau, P.; Clement, A.M.; Klembara, J.; Niedzwiedzki, G.; Berruyer, C.; Sanchez, S. New light shed on the early evolution of limb-bone growth plate and bone marrow. eLife 2021, 10, e51581.
- Belyavsky, A.; Petinati, N.; Drize, N. Hematopoiesis during ontogenesis, adult life, and aging. Int. J. Mol. Med. 2021, 22, 9231.
- Osmond, D.G. Production and selection of B lymphocytes in bone marrow: Lymphostromal interactions and apoptosis in normal, mutant and transgenic mice. Adv. Exp. Med. Biol. 1994, 355, 15–20.
- Koni, P.A.; Joshi, S.K.; Temann, U.A.; Olson, D.; Burkly, I.; Flavell, R.A. Conditional vascular cell adhesion molecule 1 depletion in mice impaired lymphocyte migration to bone marrow. J. Exp. Med. 1994, 355, 15–20.
- Abbas, A.; Lichtman, A.; Pillai, S. (Eds.) Cellular and Molecular Immunology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2022.
- Shahrabi, S.; Rezaeeyan, H.; Ahmadzadeh, A.; Shahjahani, M.; Saki, N. Bone marrow blood vessels: Normal and neoplastic niche. Oncol. Rev. 2016, 10, 72–77.
- Marenzana, M.; Arnett, T.B. The key role of the blood supply to bone. Bone Res. 2013, 1, 203–215.
- Nombela-Arrieta, C.; Manz, M.G. Quantification and three-dimensional microanatomical organization of the bone marrow. Blood Adv. 2017, 1, 407–416.
- Wu, K.; Li, R.; Zhang, Y.; Liu, Y.; Wang, M.; Huang, J.; Zhu, C.; Zhang, J.; Yuan, X.; Liu, Q. The discovery of a new type of innervation in lymphoid organs. Physiol. Rep. 2023, 11, e15604.
- Waxenbaum, J.A.; Reddy, V.; Futterman, B. Anatomy, back, thoracic vertebrae. In StatPearls (Internet); StatPearls Publishing: Treasure Islands, FL, USA, 2023; Bookshelf ID: NBK459153.
- Ordovas-Montanes, J.; Rakoff-Nahoum, S.; Huang, S.; Riol-Blanco, L.; Barreiro, O.; von Andrian, U.H. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 2015, 36, 578–604.
- Comazzetto, S.; Murphy, M.M.; Berto, S.; Jeffery, E.; Zhao, Z.; Morrison, S.J. Restricted hematopoietic progenitors and erythropoiesis require SCF from Leptin receptor+ niche cells in the bone marrow. Cell Stem Cell 2019, 24, 477–486.
- Feuerer, M.; Beckhove, P.; Garbi, N.; Mahnke, Y.; Limmer, A.; Hommel, M.; Hämmerling, G.J.; Kyewski, B.; Hamann, A.; Umansky, V.; et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 2003, 9, 1151–1157.
- Mazo, I.B.; von Andrian, U.H. Adhesion and homing of blood-borne cells in bone marrow microvessels. J. Leukoc. Biol. 1999, 66, 25–32.
- Feuerer, M.; Beckhove, P.; Bai, L.; Solomayer, E.F.; Bastert, G.; Diel, I.J.; Pedain, C.; Oberniedermayr, M.; Schirrmacher, V.; Umansky, V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat. Med. 2001, 7, 452–458.
- Feuerer, M.; Beckhove, P.; Mahnke, Y.; Hommel, M.; Kyewsky, B.; Hamann, A.; Umansky, V.; Schirrmacher, V. Bone marrow microenvironment facilitating dendritic cell: CD4 T cell interactions and maintenance of CD4 memory. Int. J. Oncol. 2004, 25, 867–876.
- Khazaie, K.; Prifti, S.; Beckhove, P.; Griesbach, A.; Russel, S.; Collins, M.; Schirrmacher, V. Persistence of dormant tumor cells in the bone marrow of tumor cell-vaccinated mice correlates with long-term immunological protection. Proc. Natl. Acad. Sci. USA 1994, 91, 7430–7434.
- Müller, M.; Gounari, F.; Prifti, S.; Hacker, H.J.; Schirrmacher, V.; Khazaie, K. EblacZ tumor dormancy in bone marrow and lymph nodes: Active control of proliferating tumor cells by CD8+ immune T cells. Cancer Res. 1998, 58, 5439–5446.
- Bai, L.; Feuerer, M.; Beckhove, P.; Umansky, V.; Schirrmacher, V. Generation of dendritic cells from human bone marrow mononuclear cells: Advantages for clinical application in comparison to peripheral blood monocyte derived cells. Int. J. Oncol. 2002, 20, 247–253.
- Feuerer, M.; Rocha, M.; Bai, L.; Umansky, V.; Solomayer, E.F.; Bastert, G.; Diel, I.J.; Schirrmacher, V. Einrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer Patients. Int. J. Cancer 2001, 92, 96–105.
- Yoshida, T.; Mei, H.; Dörner, T.; Hiepe, F.; Radbruch, A.; Fillatreau, S.; Hoyer, B.F. Memory B and memory plasma cells. Immunol. Rev. 2010, 237, 117–139.
- Slamanig, S.A.; Nolte, M.A. The bone marrow as sanctuary for plasma cells and memory T-cells: Implications for adaptive immunity and vaccinology. Cells 2021, 10, 1508.
- Schirrmacher, V. New insights into mechanisms of long-term protective anti-tumor immunity induced by cancer vaccines modified by virus infection. Biomedicines 2020, 8, 55.
- Schirrmacher, V.; Feuerer, M.; Fournier, P.; Ahlert, T.; Umansky, V.; Beckhove, P. T-cell priming in bone marrow: The potential for long-lasting protective anti-tumor immunity. Trends Mol. Med. 2003, 9, 526–534.
- Schirrmacher, V. Cancer-reactive memory T cells from bone marrow: Spontaneous induction and therapeutic potential (Review). Int. J. Oncol. 2015, 47, 2005–2016.
- Di Rosa, F.; Pabst, R. The bone marrow: A nest for migratory memory T cells. Trends Immunol. 2005, 26, 360–366.
- Mazo, I.B.; Honczarenko, M.; Leung, H.; Cavanagh, L.L.; Bonasio, R.; Weninger, W.; Engelke, K.; Xia, L.; McEver, R.P.; Koni, P.A.; et al. Bone marrow as a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 2005, 22, 259–270.
- Becker, T.C.; Coley, S.M.; Wherry, E.J.; Ahmed, R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J. Immunol. 2005, 174, 1269–1273.
- Zhang, X.; Dong, H.; Lin, W.; Voss, S.; Hinkley, L.; Westergren, M.; Tian, G.; Berry, D.; Lewellen, D.; Vile, R.G.; et al. Human bone marrow: A reservoir for “enhanced effector memory” CD8+ T cells with potent recall function. J. Immunol. 2006, 177, 6730–6737.
- Murao, A.; Oka, Y.; Tsuboi, A.; Elisseeva, O.A.; Tanaka-Harada, Y.; Fujiki, F.; Nakajima, H.; Nishida, S.; Hosen, N.; Shirakata, T.; et al. High frequencies of less differentiated and more proliferative WT1-specific CD8+ T cells in bone marrow in tumor-bearing patients: An important role of bone marrow as a secondary lymphoid organ. Cancer Sci. 2010, 101, 848–854.
- Schmitz-Winnenthal, F.H.; Volk, C.; Z’graggen, K.; Galindo, L.; Nummer, D.; Ziouta, Y.; Bucur, M.; Weitz, J.; Schirrmacher, V.; Büchler, M.W.; et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005, 65, 10079–10087.
- Choi, C.; Witzens, M.; Bucur, M.; Feuerer, M.; Sommerfeld, N.; Trojan, A.; Ho, A.; Schirrmacher, V.; Goldschmidt, H.; Beckhove, P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood 2005, 105, 2132–2134.
- Sung, J.H.; Zhang, H.; Moseman, E.A.; Alvarez, D.; Iannacone, M.; Henrickson, S.E.; de la Torre, J.C.; Groom, J.R.; Luster, A.D.; von Andrian, U.H. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 2012, 150, 1249–1263.
- Hanazawa, A.; Hayashizaki, K.; Shinoda, K.; Yagita, H.; Okumura, K.; Lohning, M.; Hara, T.; Tani-ichi, S.; Ikuta, K.; Eckes, B.; et al. CD49b-dependent establishment of T helper cell memory. Immunol. Cell Biol. 2013, 91, 524–531.
- Pascutti, M.F.; Geerman, S.; Collins, N.; Brasser, G.; Nota, B.; Stark, R.; Behr, F.; Oja, A.; Slot, E.; Panagioti, E.; et al. Peripheral and systemic antigens elicit an expandable pool of resident memory CD8+ T cells in the bone marrow. Eur. J. Immunol. 2019, 49, 853–872.
- Kudernatsch, R.F.; Letsch, A.; Guerreiro, M.; Löbel, M.; Bauer, S.; Volk, H.D.; Scheibenbogen, C. Human bone marrow contains a subset of quiescent early memory CD8+ T cells characterized by high CD127 expression and efflux capacity. Eur. J. Immunol. 2014, 44, 3532–3542.
- Wu, K.; Li, Y.; Zhang, S.; Zhou, N.; Liu, B.; Pan, T.; Zhang, X.; Luo, H.; Huang, Z.; Li, X.; et al. Preferential homing of tumor-specific and functional CD8+ stem cell-like memory T cells to the bone marrow. J. Immunother. 2019, 42, 197–207.
- Wu, K.; Wang, F.; Guo, G.; Li, Y.; Qiu, L.-J.; Li, X. CD4+ TSCMs in the bone marrow assist in maturation of antibodies against Influenza in mice. Mediat. Inflamm. 2019, 2019, 3231696.
- Chi, X.; Luo, S.; Ye, P.; Hwang, W.-L.; Cha, J.-H.; Yan, X.; Yang, W.-H. T-cell exhaustion and stemness in anti-tumor immunity: Characteristics, mechanisms, and implications. Front. Immunol. 2023, 20, 4771.
- Wang, S.; Wang, L.; Liu, Y.; Zhu, Y.; Liu, Y. Characteristics of T-cell receptor repertoire of stem cell-like memory CD4+ T cells. PeerJ. 2021, 9, e11987.
- Palendira, U.; Chinn, R.; Raza, W.; Piper, K.; Pratt, G.; Machado, L.; Bell, A.; Khan, N.; Hislop, A.D.; Steyn, R.; et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within human bone marrow. Blood 2008, 112, 3293–3302.
- Pangrazzi, L.; Naismith, E.; Meryk, A.; Keller, M.; Jenewein, B.; Trieb, K.; Grubeck-Loebenstein, B. Increased IL-15 production and accumulation of highly differentiated CD8+ effector/memory T cells in the bone marrow of persons with cytomegalovirus. Front. Immunol. 2017, 8, 715.
- DiRosa, F. Two niches in the bone marrow: A hypothesis on life-long T cell memory. Trends Immunol. 2016, 17, 503–512.
- Chang, H.-D.; Radbruch, A. Maintenance of quiescent immune memory in the bone marrow. Eur. J. Immunol. 2021, 51, 1592–1601.
- Chang, H.-D.; Tokoyoda, K.; Radbruch, A. Immunological memories of the bone marrow. Immunol. Rev. 2018, 283, 86–98.
- Rajewsky, K.; Schirrmacher, V.; Nase, S.; Jerne, N. The requirement for more than one antigenic determinant for immunogenicity. J. Exp. Med. 1969, 129, 1131–1143.
- Fresnay, S.; Zhang, X.; Strome, S.; Sewell, D.A. Bone marrow vaccination: A novel approach to enhance antigen specific antitumor immunity. Vaccine 2011, 29, 8599–8605.
- Kushida, T.; Inaba, M.; Ichioka, N.; Esumi, T.; Ogawa, R.; Iida, H.; Ikehara, S. Intra-bone marrow injection of allogeneic bone marrow cells: A powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood 2001, 97, 3292–3299.
- Wildes, T.J.; Grippin, A.; Dyson, K.A.; Wummer, B.M.; Damiani, D.; Abraham, R.S.; Flores, C.T.; Mitchell, D.A. Cross-talk between T cells and hematopoietic stem cells during adopitive cellular therapy for malignant glioma. Clin. Cancer Res. 2019, 24, 3955–3966.
- Niu, C.; Yu, J.; Zou, T.; Lu, Y.; Deng, L.; Yun, H.; Si, C.-Y.; Wu, X.; Jiang, H.; Guo, T.; et al. Identification of hematopoietic stem cells residing in the meninges of adult mice at steady state. Cell Rep. 2022, 41, 111592.
- Tsunokuma, N.; Yamane, T.; Matsumoto, C.; Tsuneto, M.; Isono, K.; Imanaka-Yoshida, K.; Yamazaki, H. Depletion of neural crest-derived cells leads to reduction in plasma noradrenaline and alters B lymphopoiesis. J. Immunol. 2017, 198, 156–169.
- Ott, L.C.; Han, C.Y.; Mueller, J.L.; Rahman, A.A.; Hotta, R.; Goldstein, A.M.; Stavely, R. Bone marrow stem cells derived from nerves have neurogenic properties and potential uitility for regenerative therapy. Int. J. Mol. Sci. 2023, 24, 5211.
- Coste, C.; Neirinckx, V.; Sharma, A.; Agirman, G.; Rogister, B.; Foguenne, J.; Lallemend, F.; Gothot, A.; Wislet, S. Human bone marrow harbors cells with neural crest-associated characteristics like human adipose and dermis tissues. PLoS ONE 2017, 12, e0177962.
- Akhter, W.; Nakhle, J.; Vaillant, L.; Garcin, G.; Le Saout, C.; Simon, M.; Crozet, C.; Djouad, F.; Jorgensen, C.; Vignais, M.-L.; et al. Transfer of mesenchymal stem cell mitochondria to CD4+ T cells contributes to repress Th1 differentiation by downregulating T-bet expression. Stem Cell Res. Ther. 2023, 14, 12.
- Schirrmacher, V. Mitochondria at work: New insights into regulation and dysregulation of cellular energy supply and metabolism. Biomedicines 2020, 8, 526.
- Nagai, M.; Noguchi, R.; Takahashi, D.; Morikawa, T.; Koshida, K.; Komiyama, S.; Ishihara, N.; Yamada, T.; Kawamura, Y.I.; Muroi, K.; et al. Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell 2019, 178, 1072–1087.
- Collins, N.; Han, S.-J.; Enamorado, M.; Link, V.M.; Huang, B.; Moseman, A.; Kishton, R.J.; Shannon, J.P.; Dixit, D.; Schwab, S.R.; et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 2019, 178, 1088–1101.
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 2019, 178, 1102–1114.
- Vollmann, E.H.; Rattay, K.; Barreiro, O.; Thiriot, A.; Fuhlbrigge, R.A.; Vrbanac, V.; Kim, K.-W.; Jung, S.; Tager, A.M.; von Andrian, U.H. Specialized transendothelial dendritic cells mediate thymic T-cell selection agains blood-borne macromolecules. Nat. Commun. 2021, 12, 6230.
- Matioubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360.
- Waller, K.M.; De La Mata, N.L.; Kelly, P.J.; Ramachandran, V.; Rawlinson, W.D.; Wyburn, K.R.; Webster, A.C. Residual risk of infection with blood-borne viruses in potential organ donors at increased risk of infection: Systematic review and meta-analysis. Med. J. Aust. 2019, 211, 414–420.
- Cariappa, A.; Mazo, I.B.; Chase, C.; Shi, H.N.; Liu, H.; Rose, H.; Leung, H.; Cherayil, B.J.; Russell, P.; von Andrian, U.; et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity 2005, 23, 397–407.
- He, S.; Ding, L.; Yuan, H.; Zhan, G.; Yang, X.; Wu, Y. A review of sensors for classification and subtype discrimination of cancer: Insights into circulating tumor cells and tumor-derived extracellular vesicles. Anal. Chim. Acta 2023, 1244, 340703.
- Rak, J.; Strzadala, L. Heterogeneity of extracellular vesicles and particles: Molecular voxels in the blood borne “hologram” of organ function, disfunction and cancer. Arch. Immunol. Ther. Exp. 2023, 71, 5.
- Li, J.; Li, J.; Hao, H.; Lu, F.; Wang, J.; Ma, M.; Jia, B.; Zhou, M.; Wang, J.; Chi, Y.; et al. Secreted proteins MDK, WFDC2, and CXCL14 as candidate biomarkers for early diagnosis of lung adenocarcinoma. BMC Cancer 2023, 23, 110.
- Tripathy, A.; John, V.; Wadden, J.; Kong, S.; Sharba, S.; Koschmann, C. Liquid biopsy in pedriatic brain tumors. Front. Genet. 2023, 13, 1114762.
- Stergiopoulou, D.; Markou, A.; Strati, A.; Zavridou, M.; Tzanikou, E.; Mastoraki, S.; Kallergi, G.; Georgoulias, V.; Lianidou, E. Comprehensive liquid biopsy analysis as a tool for the early detection of minimal residual disease in breast cancer. Sci. Rep. 2023, 13, 1258.
- Someya, M.; Hasegawa, T.; Tsuchiya, T.; Kitagawa, M.; Fukushima, Y.; Gocho, T.; Mafune, S.; Ikeuchi, Y.; Kozuka, Y.; Idogawa, M.; et al. Predictive value of an exosomal microRNA-based signature for tumor immunity in cervical cancer patients treated with chemoradiotherapy. Med. Mol. Morphol. 2023, 56, 38–45.
- Kikuchi, Y.; Tokita, S.; Hirama, T.; Kochin, V.; Nakatsugawa, M.; Shinkawa, T.; Hirohashi, Y.; Tsukahara, T.; Hata, F.; Takemasa, I.; et al. CD8+ T-cell immune surveillance against a tumor antigen encoded by the oncogenic long noncoding RNA PVT1. Cancer Immunol. Res. 2021, 9, 1342–1353.
- Cavanagh, L.L.; Bonasio, R.; Mazo, I.B.; Halin, C.; Cheng, G.; van der Welden, A.W.M.; Cariappa, A.; Chase, C.; Russell, P.; Starnbach, M.N.; et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 2005, 6, 1029–1037.
- Alvarez, D.; Vollmann, E.H.; von Andrian, U.H. Mechanisms and consequences of dendritic cell migration. Immunity 2008, 29, 325.
- Xia, Y.; Liu, A.; Li, W.; Liu, Y.; Zhang, G.; Ye, S.; Zhao, Z.; Shi, J.; Jia, Y.; Liu, X.; et al. Reference range of naïve T and T memory lymphocyte subsets in peripheral blood of healthy adult. Clin. Trial. 2022, 207, 208–217.
- Mollgard, K.; Beinlich, F.R.M.; Kusk, P.; Miyakoshi, L.M.; Delle, C.; Pla, V.; Hauglund, N.L.; Esmail, T.; Rasmussen, M.K.; Gomolka, R.S.; et al. A mesothelium divides the subarachnoid space into functional compartments. Science 2023, 378, 84–88.
- Klimov, V.; Cherevko, N.; Klimov, A.; Novikov, P. Neuronal-immune cell units in allergic inflammation in the nose. Int. J. Mol. Sci. 2022, 23, 6938.
- Ji, H.; Lai, D.; Tou, J. Neuroimmune regulation in Hirschsprung’s disease associated enterocolitis. Front. Immunol. 2023, 14, 1127375.
- Barden, M.M.; Omuro, A.M. Top advances of the year: Neuro-oncology. Cancer 2023, 129, 1467–1472.
- Mahajan, S.; Schmidt, M.H.H.; Schuman, U. The glioma immune landscape: A double-edged sword for treatment regimens. Cancers 2023, 15, 2024.
- Bunse, L.; Bunse, T.; Krämer, C.; Chih, Y.-C.; Platten, M. Clinical and translational advances in glioma immunotherapy. Neurotherapeutics 2022, 19, 1799–1817.
- Yoshimura, A.; Ohyagi, M.; Ito, M. T cells in the brain inflammation. Adv. Immunol. 2023, 157, 29–58.
- Hu, Y.; Feng, J.; Gu, T.; Wang, L.; Wang, Y.; Zhou, L.; Hong, R.; Yin, E.T.S.; Zhang, M.; Lu, P.; et al. CAR T-cell therapies in China: Rapid evolution and a bright future. Lancet Hematol. 2022, 9, e930–e941.
- Kitamura, W.; Asada, N.; Naoi, Y.; Abe, M.; Fujiwara, H.; Ennishi, D.; Nishimori, H.; Fujii, K.; Fujii, N.; Matsuoka, K.-I.; et al. Bone marrow microenvironment disruption and sustained inflammation with prolonged haematologic toxicity after CAR T-cell therapy. Br. J. Haematol. 2023, 202, 294–307.
- Tao, R.; Mi, B.; Hu, Y.; Lin, S.; Xiong, Y.; Lu, X.; Panayi, A.C.; Li, G.; Liu, G. Hallmarks of peripheral nerve function in bone regeneration. Bone Res. 2023, 11, 6.
- Manz, B.N.; Jackson, B.L.; Petit, R.S.; Dustion, M.L.; Groves, J. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell clusters. Proc. Natl. Acad. Sci. USA 2011, 108, 9089–9094.
- Henrickson, S.E.; Perro, M.; Loughhead, S.M.; Senman, B.; Stutte, S.; Quigley, M.; Alexe, G.; Iannacone, M.; Flynn, M.P.; Omid, S.; et al. Antigen availability determines CD8+ T cell-dendritic cell interaction kinetics and memory fate decisions. Immunity 2013, 39, 496–507.
- Borger, J.; Zamoyska, R.; Gakamsky, D.M. Proximity of TCR and its CD8 coreceptor controls sensitivity of T cells. Immunol. Lett. 2014, 157, 16–22.
- Sedwick, C.E.; Morgan, M.M.; Jusino, L.; Cannon, J.L.; Miller, J.; Burkhardt, J.K. TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J. Immunol. 1999, 162, 1367–1375.
- Geltink, R.I.K.; O’Sullivan, D.; Corrado, M.; Bremser, A.; Buck, M.D.; Buescher, J.M.; Firat, E.; Zhu, X.; Niedermann, G.; Caputa, G.; et al. Mitochondrial priming by CD28. Cell 2017, 171, 385–397.e11.
- Panda, A.K.; Kim, Y.; Shevach, E.M. Control of memory phenotype T lymphocyte homeostasis: The role of costimulation. J. Immunol. 2022, 208, 851–860.
- Martin-Cófreces, N.B.; Valpuesta, J.M.; Sánchez-Madrid, F. Folding for the immune synapse: CCT chaperonin and the cytoskeleton. Front. Cell Dev. Biol. 2021, 9, 658460.
- Lanzavecchia, A.; Sallusto, F. From synapses to immunological memory: The role of sustained T cell stimulation. Curr. Opin. Immunol. 2000, 12, 92–98.
- Waldman, M.M.; Rahkola, J.T.; Sigler, A.L.; Chung, J.W.; Willett, B.A.S.; Kedl, R.M.; Friedman, R.S.; Jacobelli, J. Ena/VASP protein-mediated actin polymerization contributes to naïve CD8+ T cell activation and expansion by promoting T cell-APC interactions in vivo. Front. Immunol. 2022, 13, 856977.
- Kaitao, L.; William, R.; Zhou, Y.; Cheng, Z. Single-molecule investigations on T-cell activation. Curr. Opin. Biomed. Eng. 2019, 12, 102–110.
- Cassioli, C.; Baldari, C.T. Lymphocyte polarization during immune synapse assembly: Centrosomal actin joins the game. Front. Immunol. 2022, 13, 830835.
- Mastrogiovanni, M.; Juzanz, M.; Alcover, A.; Di Bartolo, V. Coordinating cytoskeleton and molecular traffic in T cell migration, activation, and effector functions. Front. Cell Dev. Biol. 2020, 8, 591348.
- Manes, T.D.; Pober, J.S. T cell receptor-driven transendothelial migration of human effector memory CD4 T cells involves Vav, Rac, and Myosin IIA. J. Immunol. 2013, 190, 3079–3088.
- Schirrmacher, V.; Schlude, C.; Weitz, J.; Beckhove, P. Strong T-cell costimulation can reactivate tumor antigen-specific T cells in late-stage metastasized colorectal carcinoma patients: Results from a phase I clinical study. Int. J. Oncol. 2015, 46, 71–77.
- Biondi, M.; Tettamanti, S.; Galimberti, S.; Cerina, B.; Tomasoni, C.; Piazza, R.; Donsante, S.; Bido, S.; Perriello, V.M.; Broccoli, V.; et al. Selective homing of CAR-CIK cells to the bone marrow niche enhances control of the Acute myeloid leukemia burden. Blood 2023, 141, 2587–2598.
- Kim, H.-R.; Jun, C.-D. T cell microvilli: Sensors or senders? Front. Immunol. 2019, 10, 1753.
- Cornelis, R.; Hahne, S.; Taddeo, A.; Petkau, G.; Malko, D.; Durek, P.; Thiem, M.; Heiberger, L.; Peter, L.; Mohr, R.; et al. Stromal cell-contact dependent PI3K and APRIL induced NF-kB signaling prevent mitochondrial and ER stress induced death of memory plasma cells. Cell Rep. 2020, 32, 107982.
- Rozanski, C.H.; Arens, R.; Carlson, L.M.; Nair, J.; Boise, L.H.; Chanan-Khan, A.A.; Schoenberger, S.P.; Lee, K.P. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J. Exp. Med. 2011, 208, 1435–1446.
- Snell, L.M.; Lin, G.H.Y.; Watts, T.H. IL-15-dependent upregulation of GITR on CD8 memory phenotype T cells in the bone marrow relative to spleen and lymph node suggests the bone marrow as a site of superior bioavailability of IL-15. J. Immunol. 2012, 188, 5915–5923.
- Schluns, K.S.; Kieper, W.C.; Jameson, S.C.; Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000, 1, 426–432.
- Wols, H.A.M.; Underhill, G.H.; Kansas, G.S.; Witte, P.L. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol. 2002, 169, 4213–4221.
- Delacher, M.; Imbusch, C.D.; Weichenhan, D.; Breiling, A.; Wagenblatt, A.H.; Träger, U.; Hofer, A.-C.; Kägebein, D.; Wang, Q.; Frauhammer, F.; et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat. Immunol. 2017, 18, 1160–1172.
- Ashman, J.; Mutsonziwa, N.; Romano, M.M.; Kordasti, S.; Lombardi, G.; Shangaris, P. Regulatory T cell niche in the bone marrow, a new player in hematopoietic stem cell transplantation. Blood Rev. 2022, 31, 101030.
- Tikka, C.; Beasley, L.; Xu, C.; Yang, J.; Cooper, S.; Lechner, J.; Gutch, S.; Kaplan, M.H.; Capitano, M.; Yang, K. BATF sustains homeostasis and functionality of bone marrow Treg cells to preserve homeostatic regulation of hematopoiesis and development of B cells. Front. Immunol. 2023, 14, 1026368.
- Wang, X.; Hanuffa, M.A.; Holtick, U.; Collin, M.P.; Jackson, G.; Hilkens, C.M.U.; Holler, E.; Edinger, M.; Hoffmann, P.; Dickinson, A.M. Regulatory T-cell suppression of CD8+ T-cell-mediated graft-versus-host reaction requires their presence during priming. Transplantation 2009, 88, 188–197.
- Schirrmacher, V.; Beckhove, P.; Choi, C.; Griesbach, A.; Mahnke, Y. Tumor-immune memory T cells from the bone marrow exert GvL without GvH reactivity in advanced metastasized cancer. Int. J. Oncol. 2005, 27, 1141–1149.
- Brinkrolf, P.; Landmeier, S.; Altvater, B.; Chen, C.; Pscherer, S.; Rosemann, A.; Ranft, A.; Dirksen, U.; Juergens, H.; Rossig, C. A high proportion of bone marrow T cells with regulatory phenotype (CD4+CD25hiFoxP3+) in Ewing sarcoma patients is associated with metastatic disease. Int. J. Cancer 2009, 125, 879–886.
- Koch, J.; Schober, S.J.; Hindupur, S.V.; Schöning, C.; Klein, F.G.; Mantwill, K.; Ehrenfeld, M.; Schillinger, U.; Hohnecker, T.; Qi, P.; et al. Targeting the Retinoblastoma/E2F repressive complex by CDK4/6 inhibitors amplifies oncolytic potency of an oncolytic adenovirus. Nat. Commun. 2022, 13, 4689.
- Ge, Y.; Böhm, H.-H.; Rathinasamy, A.; Xydia, M.; Hu, X.; Pincha, M.; Umansky, L.; Breyer, C.; Hillier, M.; Bonertz, A.; et al. Tumor-specific regulatory T cells from the bone marrow orchestrate antitumor immunity in breast cancer. Cancer Immunol. Res. 2019, 7, 1998–2012.
- Cho, D.S.; Schmitt, R.E.; Dasgupta, A.; Ducharme, A.M.; Doles, J.D. Acute and sustained alterations to the bone marrow immune microenvironment following polymicrobial infection. Shock 2022, 58, 45–55.
- Feng, S.; Xu, Z.; Zhang, Z.; Mo, Y.; Deng, Y.; Li, L.; Fei, S.; Wu, J.; Wang, K.; Zhang, Q.; et al. RNA-Seq approach to investigate the effects of melatonin on bone marrow-derived dendritic cells from dextran sodium-induced colitis mice. Toxicology 2022, 481, 153354.
- Flores-Guzmaán, F.; Utikal, J.; Umansky, V. Dormant tumor cells interact with memory CD8+ T cells in RET transgenic mouse melanoma model. Cancer Lett. 2020, 474, 74–81.
- Mahnke, Y.D.; Schirrmacher, V. A novel tumor model system for the study of long-term protective immunity and immune T cell memory. Cell. Immunol. 2005, 221, 89–99.
- Mahnke, Y.D.; Schwendemann, J.; Beckhove, P.; Schirrmacher, V. Maintenance of lon-term tumour-specific T-cell memory by residual dormant tumour cells. Immunology 2005, 115, 325–336.
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem cells: A comprehensive review of origins and emerging clinical roles in medical practice. Orthop. Rev. 2022, 14, 37498.
- Senkal, S.; Hayal, T.B.; Sagrac, D.; Sisli, H.B.; Asutay, A.B.; Kirath, B.; Sümer, E.; Rizvanov, A.A.; Sahin, F.; Dogan, A. Human ESC-derived neuromesodermal progenitors (NMPs) successfully differentiate into mesenchymal stem cells (MSCs). Stem Cell Rev. Rep. 2022, 18, 278–293.
- Saidova, A.A.; Vorobjev, I.A. Lineage commitment, signaling pathways, and the cytoskeleton systems in mesenchymal stem cells. Tissue Eng. Part B Rev. 2020, 26, 13–25.
- Qi, S.; Zhong, Z.; Zhu, Y.; Wang, Y.; Ma, M.; Wang, Y.; Liu, X.; Jin, R.; Jiao, Z.; Zhu, R.; et al. Two Hippo signaling modules orchestrate liver size and tumorigenesis. EMBO J. 2023, 42, e112126.
- Yan, Y.-C.; Li, Y.-H.; Xiao, B.-G.; Wang, J.; Xi, J.-Y.; Yu, W.-B. Cellular and molecular mechanisms underly the combined treatment of fasudil and bone marrow derived-neuronal stem cells in a Parkinson’s disease mouse model. Mol. Neurobiol. 2023, 60, 1826–1835.
