Bone fragility is a common complication in subjects with type 2 diabetes mellitus (T2DM). However, traditional techniques for the evaluation of bone fragility, such as dual-energy X-ray absorptiometry (DXA), do not perform well in this population. Moreover, the Fracture Risk Assessment Tool (FRAX) usually underestimates fracture risk in T2DM. Importantly, novel technologies for the assessment of one microarchitecture in patients with T2DM, such as the trabecular bone score (TBS), high-resolution peripheral quantitative computed tomography (HR-pQCT), and microindentation, are emerging. Furthermore, different serum and urine bone biomarkers may also be useful for the evaluation of bone quality in T2DM.
- type 2 diabetes mellitus
- bone fragility
- fracture risk
- bone structure
- bone quality
1. Introduction
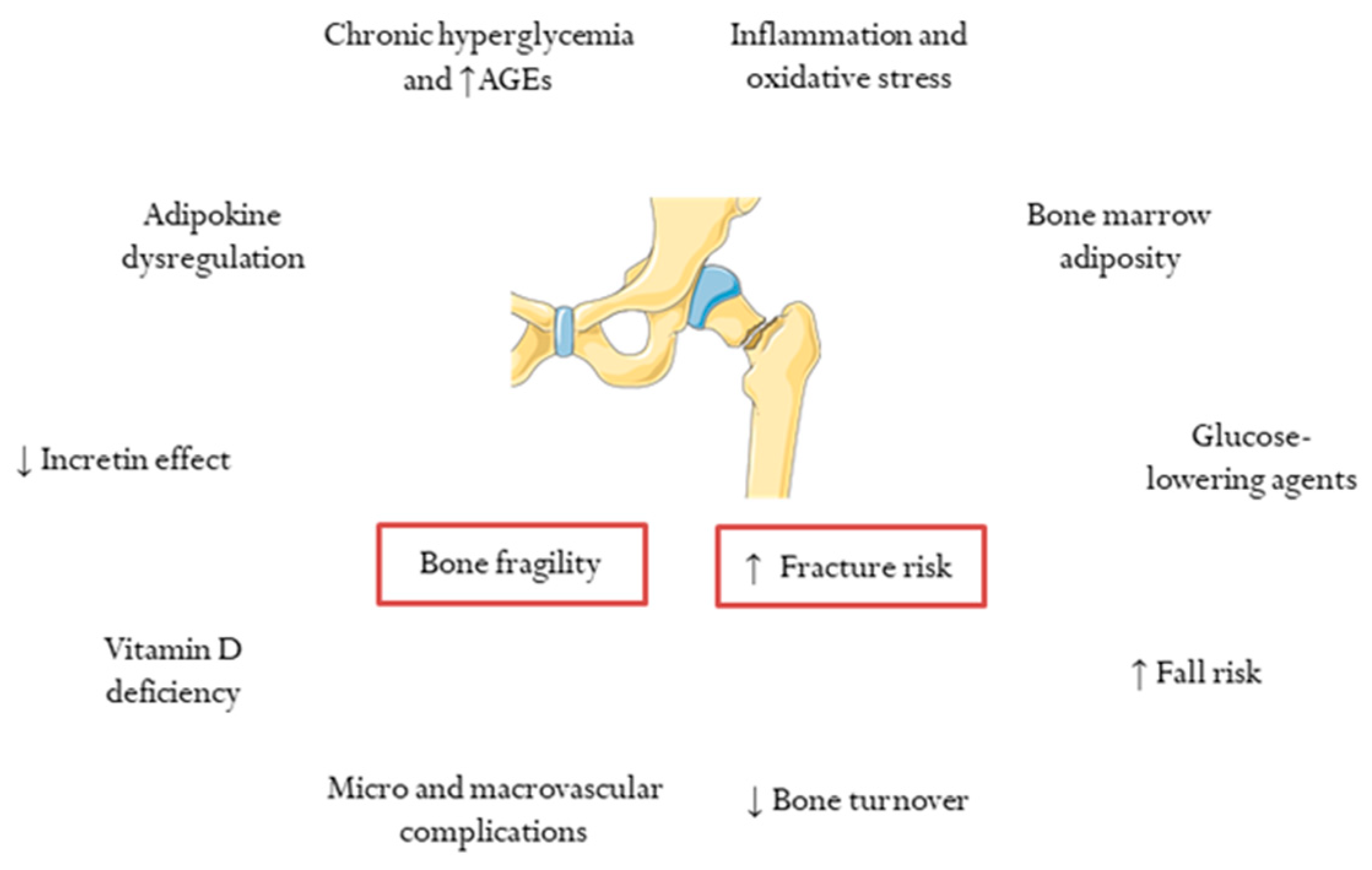
2. Determinants of Skeletal Fragility and Increased Risk of Fracture in T2DM
3. Bone Density and Fracture Risk Prediction in T2DM
4. Bone Microstructure in T2DM
5. Bone Quality in T2DM: The Role of Biomarkers of Bone Fragility
This entry is adapted from the peer-reviewed paper 10.3390/jcm11082206
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98.
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. 2), 25–32.
- Alaofè, H.; Amoussa Hounkpatin, W.; Djrolo, F.; Ehiri, J.; Rosales, C. Factors Associated with Quality of Life in Patients with Type 2 Diabetes of South Benin: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2360.
- Fenwick, E.K.; Pesudovs, K.; Khadka, J.; Dirani, M.; Rees, G.; Wong, T.Y.; Lamoureux, E.L. The impact of diabetic retinopathy on quality of life: Qualitative findings from an item bank development project. Qual. Life Res. 2012, 21, 1771–1782.
- Degu, H.; Wondimagegnehu, A.; Yifru, Y.M.; Belachew, A. Is health related quality of life influenced by diabetic neuropathic pain among type II diabetes mellitus patients in Ethiopia? PLoS ONE 2019, 14, e0211449.
- Sinjari, B.; Feragalli, B.; Cornelli, U.; Belcaro, G.; Vitacolonna, E.; Santilli, M.; Rexhepi, I.; D’Addazio, G.; Zuccari, F.; Caputi, S. Artificial Saliva in Diabetic Xerostomia (ASDIX): Double Blind Trial of Aldiamed® Versus Placebo. J. Clin. Med. 2020, 9, 2196.
- Khosla, S.; Samakkarnthai, P.; Monroe, D.G.; Farr, J.N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 685–697.
- Schousboe, J.T.; Morin, S.N.; Kline, G.A.; Lix, L.M.; Leslie, W.D. Differential risk of fracture attributable to type 2 diabetes mellitus according to skeletal site. Bone 2021, 154, 116220.
- Wang, B.; Wang, Z.; Poundarik, A.A.; Zaki, M.J.; Bockman, R.S.; Glicksberg, B.S.; Nadkarni, G.N.; Vashishth, D. Unmasking Fracture Risk in Type 2 Diabetes: The Association of Longitudinal Glycemic Hemoglobin Level and Medications. J. Clin. Endocrinol. Metab. 2021, 107, e1390–e1401.
- Schwartz, A.V. Epidemiology of fractures in type 2 diabetes. Bone 2016, 82, 2–8.
- Koromani, F.; Ghatan, S.; van Hoek, M.; Zillikens, M.C.; Oei, E.H.G.; Rivadeneira, F.; Oei, L. Type 2 Diabetes Mellitus and Vertebral Fracture Risk. Curr. Osteoporos. Rep. 2021, 19, 50–57.
- Koromani, F.; Oei, L.; Shevroja, E.; Trajanoska, K.; Schoufour, J.; Muka, T.; Franco, O.H.; Ikram, M.A.; Zillikens, M.C.; Uitterlinden, A.G.; et al. Vertebral Fractures in Individuals with Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care 2020, 43, 137–144.
- Vilaca, T.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone 2020, 137, 115457.
- Janghorbani, M.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic Review of Type 1 and Type 2 Diabetes Mellitus and Risk of Fracture. Am. J. Epidemiol. 2007, 166, 495–505.
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554.
- Jamari, J.; Ammarullah, M.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Funct. Biomater. 2021, 12, 38.
- Sato, M.; Ye, W.; Sugihara, T.; Isaka, Y. Fracture risk and healthcare resource utilization and costs among osteoporosis patients with type 2 diabetes mellitus and without diabetes mellitus in Japan: Retrospective analysis of a hospital claims database. BMC Musculoskelet. Disord. 2016, 17, 489.
- Shah, A.; Wu, F.; Jones, G.; Cicuttini, F.; Toh, L.S.; Laslett, L.L. The association between incident vertebral deformities, health-related quality of life and functional impairment: A 10.7-year cohort study. Osteoporos. Int. 2021, 32, 2247–2255.
- Peeters, C.M.M.; Visser, E.; Van de Ree, C.L.P.; Gosens, T.; Den Oudsten, B.L.; De Vries, J. Quality of life after hip fracture in the elderly: A systematic literature review. Injury 2016, 47, 1369–1382.
- Ferrari, S.L.; Abrahamsen, B.; Napoli, N.; Akesson, K.; Chandran, M.; Eastell, R.; El-Hajj Fuleihan, G.; Josse, R.; Kendler, D.L.; Kraenzlin, M.; et al. Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos. Int. 2018, 29, 2585–2596.
- de Waard, E.A.C.; van Geel, T.A.C.M.; Savelberg, H.H.C.M.; Koster, A.; Geusens, P.P.M.M.; van den Bergh, J.P.W. Increased fracture risk in patients with type 2 diabetes mellitus: An overview of the underlying mechanisms and the usefulness of imaging modalities and fracture risk assessment tools. Maturitas 2014, 79, 265–274.
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220.
- Majumdar, S.R.; Leslie, W.D.; Lix, L.M.; Morin, S.N.; Johansson, H.; Oden, A.; McCloskey, E.V.; Kanis, J.A. Longer Duration of Diabetes Strongly Impacts Fracture Risk Assessment: The Manitoba BMD Cohort. J. Clin. Endocrinol. Metab. 2016, 101, 4489–4496.
- Dufour, A.B.; Kiel, D.P.; Williams, S.A.; Weiss, R.J.; Samelson, E.J. Risk Factors for Incident Fracture in Older Adults with Type 2 Diabetes: The Framingham Heart Study. Diabetes Care 2021, 44, 1547–1555.
- Li, C.-I.; Liu, C.-S.; Lin, W.-Y.; Meng, N.-H.; Chen, C.-C.; Yang, S.-Y.; Chen, H.-J.; Lin, C.-C.; Li, T.-C. Glycated Hemoglobin Level and Risk of Hip Fracture in Older People with Type 2 Diabetes: A Competing Risk Analysis of Taiwan Diabetes Cohort Study. J. Bone Miner. Res. 2015, 30, 1338–1346.
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.S.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.G.; van Meurs, J.B.J.; Janssen, J.A.M.J.L.; et al. High Bone Mineral Density and Fracture Risk in Type 2 Diabetes as Skeletal Complications of Inadequate Glucose Control: The Rotterdam Study. Diabetes Care 2013, 36, 1619–1628.
- Romero-Díaz, C.; Duarte-Montero, D.; Gutiérrez-Romero, S.A.; Mendivil, C.O. Diabetes and Bone Fragility. Diabetes Ther. 2020, 12, 71–86.
- Starup-Linde, J.; Vestergaard, P. Biochemical bone turnover markers in diabetes mellitus—A systematic review. Bone 2016, 82, 69–78.
- Tonks, K.T.; White, C.; Center, J.R.; Samocha-Bonet, D.; Greenfield, J. Bone Turnover Is Suppressed in Insulin Resistance, Independent of Adiposity. J. Clin. Endocrinol. Metab. 2017, 102, 1112–1121.
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219.
- Nuche-Berenguer, B.; Portal-Núñez, S.; Moreno, P.; González, N.; Acitores, A.; López-Herradón, A.; Esbrit, P.; Valverde, I.; Villanueva-Peñacarrillo, M.L. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J. Cell. Physiol. 2010, 225, 585–592.
- Muñoz-Torres, M.; Reyes-García, R.; García-Martin, A.; Jiménez-Moleón, J.J.; Gonzalez-Ramírez, A.R.; Lara-Villoslada, M.J.; Moreno, P.R. Ischemic heart disease is associated with vertebral fractures in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2013, 4, 310–315.
- Mitri, J.; Pittas, A.G. Vitamin D and Diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 205–232.
- Knudsen, J.K.; Leutscher, P.; Sørensen, S. Gut Microbiota in Bone Health and Diabetes. Curr. Osteoporos. Rep. 2021, 19, 462–479.
- Shanbhogue, V.V.; Mitchell, D.M.; Rosen, C.J.; Bouxsein, M.L. Type 2 diabetes and the skeleton: New insights into sweet bones. Lancet Diabetes Endocrinol. 2016, 4, 159–173.
- Rozas-Moreno, P.; Reyes-García, R.; Jódar-Gimeno, E.; Varsavsky, M.; Luque-Fernández, I.; Cortés-Berdonces, M.; Muñoz-Torres, M. Recomendaciones sobre el efecto de los fármacos antidiabéticos en el hueso. Endocrinol. Diabetes Nutr. 2017, 64, 1–6.
- Molinuevo, M.S.; Schurman, L.; McCarthy, A.D.; Cortizo, A.M.; Tolosa, M.J.; Gangoiti, M.V.; Arnol, V.; Sedlinsky, C. Effect of metformin on bone marrow progenitor cell differentiation: In vivo and in vitro studies. J. Bone Miner. Res. 2010, 25, 211–221.
- Monami, M.; Dicembrini, I.; Antenore, A.; Mannucci, E. Dipeptidyl Peptidase-4 Inhibitors and Bone Fractures: A meta-analysis of randomized clinical trials. Diabetes Care 2011, 34, 2474–2476.
- Su, B.; Sheng, H.; Zhang, M.; Bu, L.; Yang, P.; Li, L.; Li, F.; Sheng, C.; Han, Y.; Qu, S.; et al. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: A meta-analysis of randomized controlled trials. Endocrine 2014, 48, 107–115.
- Zhu, Z.-N.; Jiang, Y.-F.; Ding, T. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials. Bone 2014, 68, 115–123.
- Kohan, D.E.; Fioretto, P.; Tang, W.; List, J.F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014, 85, 962–971.
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657.
- Johnston, S.S.; Conner, C.; Aagren, M.; Ruiz, K.; Bouchard, J. Association between hypoglycaemic events and fall-related fractures in Medicare-covered patients with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 634–643.
- Mayne, D.; Stout, N.R.; Aspray, T.J. Diabetes, falls and fractures. Age Ageing 2010, 39, 522–525.
- Dennison, E.M.; Syddall, H.E.; Aihie Sayer, A.; Craighead, S.; Phillips, D.I.W.; Cooper, C. Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: Evidence for an indirect effect of insulin resistance? Diabetologia 2004, 47, 1963–1968.
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K. Risk of Fracture in Women with Type 2 Diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410.
- Mitchell, A.; Fall, T.; Melhus, H.; Wolk, A.; Michaëlsson, K.; Byberg, L. Type 2 Diabetes in Relation to Hip Bone Density, Area, and Bone Turnover in Swedish Men and Women: A Cross-Sectional Study. Calcif. Tissue Int. 2018, 103, 501–511.
- Ma, L.; Oei, L.; Jiang, L.; Estrada, K.; Chen, H.; Wang, Z.; Yu, Q.; Zillikens, M.C.; Gao, X.; Rivadeneira, F. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur. J. Epidemiol. 2012, 27, 319–332.
- Pan, H.; Wu, N.; Yang, T.; He, W. Association between bone mineral density and type 1 diabetes mellitus: A meta-analysis of cross-sectional studies. Diabetes Metab. Res. Rev. 2014, 30, 531–542.
- Srikanthan, P.; Crandall, C.J.; Miller-Martinez, D.; Seeman, T.E.; Greendale, G.A.; Binkley, N.; Karlamangla, A.S. Insulin Resistance and Bone Strength: Findings From the Study of Midlife in the United States. J. Bone Miner. Res. 2014, 29, 796–803.
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113.
- Botella Martínez, S.; Varo Cenarruzabeitia, N.; Escalada San Martin, J.; Calleja Canelas, A. The diabetic paradox: Bone mineral density and fracture in type 2 diabetes. Endocrinol. Nutr. 2016, 63, 495–501.
- Giangregorio, L.M.; Leslie, W.D.; Lix, L.M.; Johansson, H.; Oden, A.; McCloskey, E.; Kanis, J.A. FRAX underestimates fracture risk in patients with diabetes. J. Bone Miner. Res. 2012, 27, 301–308.
- El Miedany, Y. FRAX: Re-adjust or re-think. Arch. Osteoporos. 2020, 15, 150.
- Valentini, A.; Cianfarani, M.A.; De Meo, L.; Morabito, P.; Romanello, D.; Tarantino, U.; Federici, M.; Bertoli, A. FRAX tool in type 2 diabetic subjects: The use of HbA1c in estimating fracture risk. Acta Diabetol. 2018, 55, 1043–1050.
- Wen, Z.; Ding, N.; Chen, R.; Liu, S.; Wang, Q.; Sheng, Z.; Liu, H. Comparison of methods to improve fracture risk assessment in chinese diabetic postmenopausal women: A case-control study. Endocrine 2021, 73, 209–216.
- Hu, L.; Li, T.; Zou, Y.; Yin, X.-L.; Gan, H. The Clinical Value of the RA-Adjusted Fracture Risk Assessment Tool in the Fracture Risk Prediction of Patients with Type 2 Diabetes Mellitus in China. Int. J. Gen. Med. 2021, 14, 327–333.
- Leslie, W.D.; Johansson, H.; McCloskey, E.V.; Harvey, N.C.; Kanis, J.A.; Hans, D. Comparison of Methods for Improving Fracture Risk Assessment in Diabetes: The Manitoba BMD Registry. J. Bone Miner. Res. 2018, 33, 1923–1930.
- Hunt, H.B.; Torres, A.M.; Palomino, P.M.; Marty, E.; Saiyed, R.; Cohn, M.; Jo, J.; Warner, S.; Sroga, G.E.; King, K.B.; et al. Altered Tissue Composition, Microarchitecture, and Mechanical Performance in Cancellous Bone From Men With Type 2 Diabetes Mellitus. J. Bone Miner. Res. 2019, 34, 1191–1206.
- Starup-Linde, J.; Lykkeboe, S.; Handberg, A.; Vestergaard, P.; Høyem, P.; Fleischer, J.; Hansen, T.K.; Poulsen, P.L.; Laugesen, E. Glucose variability and low bone turnover in people with type 2 diabetes. Bone 2021, 153, 116159.
- Starup-Linde, J.; Eriksen, S.A.; Lykkeboe, S.; Handberg, A.; Vestergaard, P. Biochemical markers of bone turnover in diabetes patients—A meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos. Int. 2014, 25, 1697–1708.
- Reyes-Garcia, R.; Rozas-Moreno, P.; López-Gallardo, G.; Garcia-Martin, A.; Varsavsky, M.; Avilés-Pérez, M.D.; Muñoz-Torres, M. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2013, 50, 47–52.
- Yamamoto, M.; Yamaguchi, T.; Nawata, K.; Yamauchi, M.; Sugimoto, T. Decreased PTH Levels Accompanied by Low Bone Formation Are Associated with Vertebral Fractures in Postmenopausal Women with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 1277–1284.
- Napoli, N.; Conte, C.; Eastell, R.; Ewing, S.K.; Bauer, D.C.; Strotmeyer, E.S.; Black, D.M.; Samelson, E.J.; Vittinghoff, E.; Schwartz, A.V. Bone Turnover Markers Do Not Predict Fracture Risk in Type 2 Diabetes. J. Bone Miner. Res. 2020, 35, 2363–2371.
- Karim, L.; Moulton, J.; Van Vliet, M.; Velie, K.; Robbins, A.; Malekipour, F.; Abdeen, A.; Ayres, D.; Bouxsein, M.L. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone 2018, 114, 32–39.
- Wölfel, E.M.; Jähn-Rickert, K.; Schmidt, F.N.; Wulff, B.; Mushumba, H.; Sroga, G.E.; Püschel, K.; Milovanovic, P.; Amling, M.; Campbell, G.M.; et al. Individuals with type 2 diabetes mellitus show dimorphic and heterogeneous patterns of loss in femoral bone quality. Bone 2020, 140, 115556.
