Organotin (IV) dithiocarbamate has recently received attention as a therapeutic agent among organotin (IV) compounds. The individual properties of the organotin (IV) and dithiocarbamate moieties in the hybrid complex form a synergy of action that stimulates increased biological activity. Organotin (IV) components have been shown to play a crucial role in cytotoxicity. The biological effects of organotin compounds are believed to be influenced by the number of Sn-C bonds and the number and nature of alkyl or aryl substituents within the organotin structure. Ligands target and react with molecules while preventing unwanted changes in the biomolecules. Organotin (IV) dithiocarbamate compounds have also been shown to have a broad range of cellular, biochemical, and molecular effects, with their toxicity largely determined by their structure.
- organotin (IV)
- dithiocarbamate
- synthesis
- characterization
- cytotoxicity
1. Background Chemistry of Organotin (IV) Dithiocarbamate
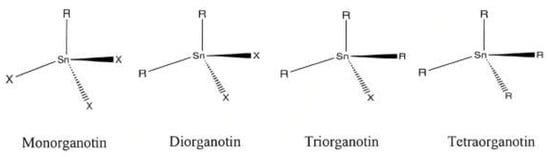
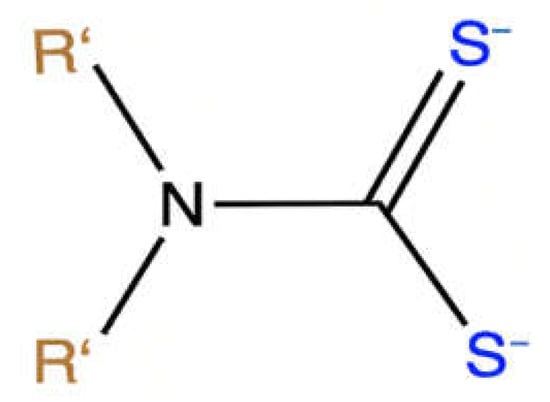
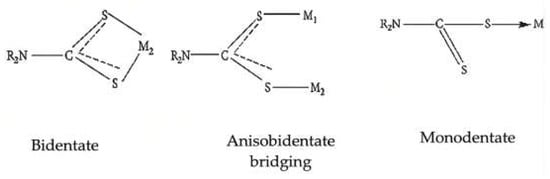
2. Synthesis of Organotin (IV) Dithiocarbamate
The in situ method has been proposed as the best way to prepare the dithiocarbamate compounds because the ligand cannot be synthesized in a solid form at a temperature above 4 °C [11]. The reaction between carbon disulfide and secondary amines is exothermic (heat release) during the production of dithiocarbamic acid [24][25]. Higher temperatures cause the ligand to break down, resulting in the formation of carbon dioxide, hydrogen sulfide, and ammonium thiocyanide [11]. Domazetis, Magee, and James (1977) prepared triphenyltin (IV) dithiocarbamate compounds using a low-temperature method, resulting in a good yield and high purity of compounds [21]. This shows that the temperature highly influences the form of the product. However, these complexes are very stable at ambient temperatures and begin to melt at temperatures exceeding 100 °C [11].
Previous studies reported that the yield using this method was greater than 50% [17][24][26][27][28][29][30]. Awang et al. and Muthalib and Baba noted in their studies that the dithiocarbamate ligands were synthesized by the nucleophilic addition of carbon disulfide to the corresponding amines in cold ethanol solutions (<4 °C) [31][32]. Generally, the addition of reagents (amines, bases, and carbon disulfide) for the synthesis of dithiocarbamates does not affect the product formed provided that the correct stoichiometric proportions are used [22]. The synthesis of the organotin (IV) dithiocarbamate complex was achieved by adding a defined amount of organotin (IV) chloride dropwise to a stirred mixture of ligands. The white precipitate that developed at the end of the process was filtered, washed with ethanol, and vacuum-dried in a desiccator over silica gel [31][32]. The compounds formed were washed with cold ethanol to remove unwanted residues from the desired product [22]. The narrow melting point intervals of approximately 1–2 °C indicated good compound purity [17].
3. Anticancer Effect of Organotin (IV) Dithiocarbamate
| Compound | IC50 Values (μM) | Tumor Cell Lines | References |
|---|---|---|---|
| Dibutyltin (IV) N-butyl-N-phenyldithiocarbamate | 0.8 | Jurkat E6.1 | [17] |
| Diphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 1.3 | ||
| Triphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 0.4 | ||
| Doxorubicin hydrochloride (control) | 0.1 | ||
| Dibutyltin (IV) N-butyl-N-phenyldithiocarbamate | 5.3 | K-562 | |
| Diphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 9.2 | ||
| Triphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 1.9 | ||
| Doxorubicin hydrochloride (control) | 11.0 | ||
| Triphenyltin (IV) benzylisopropyldithiocarbamate | 0.18 | Jurkat E6.1 | [46] |
| Triphenyltin (IV) methylisopropyldithiocarbamate | 0.03 | ||
| Triphenyltin (IV) ethylisopropyldithiocarbamate | 0.42 | ||
| Etoposide (control) | 0.12 | ||
| MeSnClL2 | >4000 | HeLa | [52] |
| BuSnClL2 | 8.12 | ||
| PhSnClL2 | 4.37 | ||
| Me2SnL2 | 12.30 | ||
| Bu2SnL2 | 11.75 | ||
| Ph2SnL2 | 0.01 | ||
| 5-Fluorouracil (control) | 40 | ||
| Dimethyltin (IV) benzyldithiocarbamate | 40 | Hela | [26] |
| Dibutyltin (IV) benzyldithiocarbamate | 0.019 | ||
| Diphenyltin (IV) benzyldithiocarbamate | 330 | ||
| 5-Fluorouracil (control) | 40 | ||
| Dimethyltin (IV) benzyldithiocarbamate | 185 | MCF-7 | |
| Dibutyltin (IV) benzyldithiocarbamate | 57.3 | ||
| Diphenyltin (IV) benzyldithiocarbamate | 20 | ||
| 5-Fluorouracil (control) | 56.2 | ||
| Diphenyltin (IV) diallyldithiocarbamate | 2.36 | HT-29 | [24] |
| Triphenyltin (IV) diallyldithiocarbamate | 0.39 | ||
| Ph3Sn(N,N-diisopropyldithiocarbamate) (OC2) | 0.55 | K562 | [47] |
| Ph3Sn(N,N-diallyldithiocarbamate) (OC4) | 1.1 | ||
| Imatinib mesylate (control) | 34 | ||
| Diphenytin (IV) N-methyl-N-hydroxyethyldithiocarbamate | 1.630 | PC-3 | [55] |
| 4.937 | Caco-2 | ||
| Camptothecin (control) | 24.41 | PC-3 | |
| >100 | Caco-2 | ||
| Triphenyltin (IV) diisopropyldithiocarbamate (ODTC 3) | 0.67 | Jurkat E6.1 | [48] |
| Triphenyltin (IV) diethyldithiocarbamate (ODTC 5) | 0.92 | ||
| Vincristine (control) | 0.24 |
| Category | IC50 Value (μg cm−3) |
|---|---|
| Highly toxic | <5.0 |
| Moderately toxic | 5.0 ≤≤ 10.0 |
| Slightly toxic | 10.0–25.0 |
| Non-toxic | >25.0 |
This entry is adapted from the peer-reviewed paper 10.3390/molecules28155841
References
- Rabiee, N.; Safarkhani, M.; Amini, M.M. Investigating the Structural Chemistry of Organotin(IV) Compounds: Recent Advances. Rev. Inorg. Chem. 2019, 39, 13–45.
- Ali, M.; Yousif, E. Chemistry and Applications of Organotin(IV) Complexes: A Review. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2611–2619.
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin(IV) Dithiocarbamate Complexes: Chemistry and Biological Activity. Molecules 2018, 23, 2571.
- Syed Annuar, S.N.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Cellular Basis of Organotin(IV) Derivatives as Anticancer Metallodrugs: A Review. Front. Chem. 2021, 9, 657599.
- Iqbal, H.; Ali, S.; Shahzadi, S. Antituberculosis Study of Organotin(IV) Complexes: A Review. Cogent Chem. 2015, 1, 1029039.
- Sunday, A.O.; Alafara, B.A.; Oladele, O.G. Toxicity and Speciation Analysis of Organotin Compounds. Chem. Speciat. Bioavailab. 2012, 24, 216–226.
- Ayanda, O.S.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J. Fate and Remediation of Organotin Compounds in Seawaters and Soils. Chem. Sci. Trans. 2012, 1, 470–481.
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological Activity Studies on Organotin(IV)N+ Complexes and Parent Compounds. J. Organomet. Chem. 2006, 691, 1733–1747.
- Carraher, C.E.; Roner, M.R. Organotin Polyethers as Biomaterials. Materials 2009, 2, 1558–1598.
- Awang, N.; Kamaludin, N.F.; Ghazali, A.R. Cytotoxic Effect of Organotin(IV) Benzylisopropyldithiocarbamate Compounds on Chang Liver Cell and Hepatocarcinoma HepG2 Cell. Pak. J. Biol. Sci. 2011, 14, 768–774.
- Kamaludin, N.F.; Awang, N. Synthesis and Characterisation of Organotin(IV) Nethyl- N-Phenyldithiocarbamate Compounds and the Crystal Structures of Dibutyl- And Triphenyltin(IV) Nethyl- N-Phenyldithiocarbamate. Res. J. Chem. Environ. 2014, 18, 90–98.
- Sainorudin, M.H.; Sidek, N.M.; Ismail, N.; Rozaini, M.Z.H.; Harun, N.A.; Tuan Anuar, T.N.S.; Azmi, A.A.A.R.; Yusoff, F. Synthesis, Characterization and Biological Activity of Organotin(IV) Complexes Featuring Di-2-Ethylhexyldithiocarbamate and N-Methylbutyldithiocarbamate as Ligands. GSTF J. Chem. Sci. 2015, 2, 2.
- Sharma, R.; Kaushik, N.K. Thermal Studies on Some Organotin(IV) Complexes with Piperidine and 2-Aminopyridine Dithiocarbamates. J. Therm. Anal. Calorim. 2004, 78, 953–964.
- Awang, N.; Baba, I. Diorganotin(IV) Alkylcyclohexyldithiocarbamate Compounds: Synthesis, Characterization and Biological Activities. Sains Malays. 2012, 41, 977–982.
- Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors 2020, 11, 12.
- Awang, N.; Baba, I.; Yamin, B.M.; Halim, A.A. Preparation, Characterization and Antimicrobial Assay of 1,10-Phenanthroline and 2,2’-Bipyridyl Adducts of Cadmium(II) N-Sec-Butyl-N- Propyldithiocarbamate: Crystal Structure of Cd2(2,2’-Bipyridyl). World Appl. Sci. J. 2011, 12, 1568–1574.
- Kamaludin, N.F.; Awang, N.; Baba, I.; Hamid, A.; Meng, C.K. Synthesis, Characterization and Crystal Structure of Organotin(IV) N-Butyl-N-Phenyldithiocarbamate Compounds and Their Cytotoxicity in Human Leukemia Cell Lines. Pak. J. Biol. Sci. 2013, 16, 12–21.
- Nabipour, H.; Ghammamy, S.; Ashuri, S.; Aghbolagh, Z.S. Synthesis of a New Dithiocarbamate Compound and Study of Its Biological Properties. Org. Chem. J. 2010, 2, 75–80.
- Jung, O.-S.; Sohn, Y.-S. Coordination Chemistry of Organotin(IV) Dithiocarbamate Complexes. Bull. Korean Chem. Soc. 1988, 9, 365–368.
- Xu, L.Z.; Zhao, P.S.; Zhang, S.S. Crystal Structure and Characterization of Pd(II) Bis(Diisopropyldithiocarbamate) Complex. Chin. J. Chem. 2001, 19, 436–440.
- Domazetis, G.; Magee, R.J.; James, B.D. Synthesis and Structure of Some Triphenyltin(IV) Dithiocarbamate Compounds. J. Organomet. Chem. 1977, 141, 57–69.
- Odularu, A.T.; Ajibade, P.A. Dithiocarbamates: Challenges, Control, and Approaches to Excellent Yield, Characterization, and Their Biological Applications. Bioinorg. Chem. Appl. 2019, 2019, 826049.
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis and Characterization of Metal Complexes of N-Alkyl-N-Phenyl Dithiocarbamates. Polyhedron 2010, 29, 1431–1436.
- Haezam, F.N.; Awang, N.; Kamaludin, N.F.; Mohamad, R. Synthesis and Cytotoxic Activity of Organotin(IV) Diallyldithiocarbamate Compounds as Anticancer Agent towards Colon Adenocarcinoma Cells (HT-29). Saudi J. Biol. Sci. 2021, 28, 3160–3168.
- Baba, I.; Raya, I. Kompleks Praseodimium Ditiokarbamat 1,10 Fenantrolin. Sains Malays. 2010, 39, 45–50.
- Adeyemi, J.O.; Onwudiwe, D.C.; Nundkumar, N.; Singh, M. Diorganotin(IV) Benzyldithiocarbamate Complexes: Synthesis, Characterization, and Thermal and Cytotoxicity Study. Open Chem. 2020, 18, 453–462.
- Adeyemi, J.O.; Onwudiwe, D.C.; Hosten, E.C. Organotin(IV) Complexes Derived from N-Ethyl-N-Phenyldithiocarbamate: Synthesis, Characterization and Thermal Studies. J. Saudi Chem. Soc. 2018, 22, 427–438.
- Adeyemi, J.O.; Onwudiwe, D.C.; Hosten, E.C. Synthesis, Characterization and the Use of Organotin(IV) Dithiocarbamate Complexes as Precursor to Tin Sulfide Nanoparticles by Heat up Approach. J. Mol. Struct. 2019, 1195, 395–402.
- Adli, H.K.; Sidek, N.M.; Ismail, N.; Khairul, W.M. Several Organotin (IV) Complexes Featuring 1-Methylpiperazinedithiocarbamate and N-Methylcyclohexyldithiocarbamate as Ligands and Their Anti-Microbial Activity Studies. Chiang Mai J. Sci. 2013, 40, 117–125.
- Mohamad, R.; Awang, N.; Farahana Kamaludin, N. Synthesis and Characterisation of New Organotin (IV)(2-Methoxyethyl)-Methyldithiocarbamate Complexes. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1920–1925.
- Awang, N.; Baba, I.; Yamin, B.M.; Othman, M.S.; Kamaludin, N.F. Synthesis, Characterization and Biological Activities of Organotin (IV) Methylcyclohexyldithiocarbamate Compounds. Am. J. Appl. Sci. 2011, 8, 310–317.
- Muthalib, A.F.A.; Baba, I. New Mono-Organotin (IV) Dithiocarbamate Complexes. In Proceedings of the AIP Conference Proceedings, Selangor, Malaysia, 9–11 April 2014; Volume 1614, pp. 237–243.
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917.
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378.
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653.
- Hadi, A.G.; Jawad, K.; Ahmed, D.S.; Yousif, E. Synthesis and Biological Activities of Organotin (IV) Carboxylates: A Review. Syst. Rev. Pharm. 2019, 10, 26–31.
- Gielen, M. Organotin Compounds and Their Therapeutic Potential: A Report from the Organometallic Chemistry Department of the Free University of Brussels. Appl. Organomet. Chem. 2002, 16, 481–494.
- Hamid, A.; Azmi, M.A.; Rajab, N.F.; Awang, N.; Jufri, N.F. Cytotoxic Effects of Organotin(IV) Dithiocarbamate Compounds with Different Functional Groups on Leukemic Cell Line, K-562. Sains Malays. 2020, 49, 1421–1430.
- Attanzio, A.; D’Agostino, S.; Busà, R.; Frazzitta, A.; Rubino, S.; Girasolo, M.A.; Sabatino, P.; Tesoriere, L. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules 2020, 25, 859.
- Gómez-Ruiz, S.; Kaluderović, G.N.; Prashar, S.; Hey-Hawkins, E.; Erić, A.; Žižak, Ž.; Juranić, Z.D. Study of the Cytotoxic Activity of Di and Triphenyltin(IV) Carboxylate Complexes. J. Inorg. Biochem. 2008, 102, 2087–2096.
- Varela-Ramirez, A.; Costanzo, M.; Carrasco, Y.P.; Pannell, K.H.; Aguilera, R.J. Cytotoxic Effects of Two Organotin Compounds and Their Mode of Inflicting Cell Death on Four Mammalian Cancer Cells. Cell Biol. Toxicol. 2011, 27, 159–168.
- Muhammad, N.; Ahmad, M.; Sirajuddin, M.; Ali, Z.; Tumanov, N.; Wouters, J.; Chafik, A.; Solak, K.; Mavi, A.; Muhammad, S.; et al. Synthesis, Characterization, Biological Activity and Molecular Docking Studies of Novel Organotin(IV) Carboxylates. Front. Pharmacol. 2022, 13, 864336.
- Koch, B.; Basu Baul, T.S.; Chatterjee, A. P53-Dependent Antiproliferative and Antitumor Effect of Novel Alkyl Series of Diorganotin(IV) Compounds. Investig. New Drugs 2009, 27, 319–326.
- Ray, D.; Sarma, K.D.; Antony, A. Differential Effects of Tri-n-Butylstannyl Benzoates on Induction of Apoptosis in K562 and MCF-7 Cells. IUBMB Life 2000, 49, 519–525.
- Wada, O.; Manabe, S.; Iwai, H.; Arakawa, Y. Recent Progress in the Study of Analytical Methods, Toxicity, Metabolism and Health Effects of Organotin Compounds. Sangyo Igaku 1982, 24, 24–54.
- Awang, N.; Yousof, N.S.A.M.; Rajab, N.F.; Kamaludin, N.F. In Vitro Cytotoxic Activity of New Triphenyltin (IV) Alkyl-Isopropyldi-Thiocarbamate Compounds on Human Acute T-Lymphoblastic Cell Line. J. Appl. Pharm. Sci. 2015, 5, 7–11.
- Syed Annuar, S.N.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Triphenyltin(IV) Dithiocarbamate Compound Induces Genotoxicity and Cytotoxicity in K562 Human Erythroleukemia Cells Primarily via Mitochondria-Mediated Apoptosis. Food Chem. Toxicol. 2022, 168, 113336.
- Rasli, N.R.; Hamid, A.; Awang, N.; Kamaludin, N.F. Series of Organotin(IV) Compounds with Different Dithiocarbamate Ligands Induced Cytotoxicity, Apoptosis and Cell Cycle Arrest on Jurkat E6.1, T Acute Lymphoblastic Leukemia Cells. Molecules 2023, 28, 3376.
- Adeyemi, J.O.; Onwudiwe, D.C.; Ekennia, A.C.; Okafor, S.N.; Hosten, E.C. Organotin(IV)N-Butyl-N-Phenyldithiocarbamate Complexes: Synthesis, Characterization, Biological Evaluation and Molecular Docking Studies. J. Mol. Struct. 2019, 1192, 15–26.
- Awang, N.; Kamaludin, N.F.; Baba, I.; Chan, K.M.; Rajaajab, N.F.; Hamid, A. Synthesis, Characterization and Antitumor Activity of New Organotin(IV) Methoxyethyldithiocarbamate Complexes. Orient. J. Chem. 2016, 32, 101–107.
- Nath, M. Toxicity and the Cardiovascular Activity of Organotin Compounds: A Review. Appl. Organomet. Chem. 2008, 22, 598–612.
- Adeyemi, J.O.; Onwudiwe, D.C. Antimicrobial and Cytotoxicity Studies of Some Organotin(IV) N-Ethyl-N-Phenyl Dithiocarbamate Complexes. Pol. J. Environ. Stud. 2020, 29, 2525–2532.
- Adeyemi, J.O.; Onwudiwe, D.C.; Singh, M. Synthesis, Characterization, and Cytotoxicity Study of Organotin(IV) Complexes Involving Different Dithiocarbamate Groups. J. Mol. Struct. 2019, 1179, 366–375.
- Tiekink, E.R.T. Tin Dithiocarbamates: Applications and Structures. Appl. Organomet. Chem. 2008, 22, 533–550.
- Adeyemi, J.O.; Olasunkanmi, L.O.; Fadaka, A.O.; Sibuyi, N.R.S.; Oyedeji, A.O.; Onwudiwe, D.C. Synthesis, Theoretical Calculation, and Biological Studies of Mono-and Diphenyltin(IV) Complexes of N-Methyl-N-Hydroxyethyldithiocarbamate. Molecules 2022, 27, 2947.
- How, F.N.-F.; Crouse, K.A.; Tahir, M.I.M.; Tarafder, M.T.H.; Cowley, A.R. Synthesis, Characterization and Biological Studies of S-Benzyl-β-N-(Benzoyl) Dithiocarbazate and Its Metal Complexes. Polyhedron 2008, 27, 3325–3329.
- Kadu, R.; Roy, H.; Singh, V.K. Diphenyltin(IV) Dithiocarbamate Macrocyclic Scaffolds as Potent Apoptosis Inducers for Human Cancer HEP 3B and IMR 32 Cells: Synthesis, Spectral Characterization, Density Functional Theory Study and in Vitro Cytotoxicity. Appl. Organomet. Chem. 2015, 29, 746–755.
- Awang, N.; Baba, I.; Mohd Yousof, N.S.A.; Kamaludin, N.F. Synthesis and Characterization of Organotin(IV) N-Benzyl-N-Isopropyldithiocarbamate Compounds: Cytotoxic Assay on Human Hepatocarcinoma Cells (HepG2). Am. J. Appl. Sci. 2010, 7, 1047–1052.
- Khalilov, R. A Comprehensive Review of Advanced Nano-Biomaterials in Regenerative Medicine and Drug Delivery. Adv. Biol. Earth Sci. 2023, 8, 5–18.
- Keskin, C.; Ölçekçi, A.; Baran, A.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Khalilov, R.; Aliyev, E.; Sufianov, A.; Beilerli, A.; et al. Green Synthesis of Silver Nanoparticles Mediated Diospyros Kaki L. (Persimmon): Determination of Chemical Composition and Evaluation of Their Antimicrobials and Anticancer Activities. Front. Chem. 2023, 11, 1187808.
- Paredes, K.O.; Díaz-García, D.; García-Almodóvar, V.; Chamizo, L.L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187.
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022, 11, e2100639.
- Corvo, M.L.; Mendo, A.S.; Figueiredo, S.; Gaspar, R.; Larguinho, M.; Guedes da Silva, M.F.C.; Baptista, P.V.; Fernandes, A.R. Liposomes as Delivery System of a Sn(IV) Complex for Cancer Therapy. Pharm. Res. 2016, 33, 1351–1358.
- Galanski, M.S.; Jakupec, M.A.; Keppler, B.K. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094.
- Cvek, B.; Dvorak, Z. Targeting of Nuclear Factor-KappaB and Proteasome by Dithiocarbamate Complexes with Metals. Curr. Pharm. Des. 2007, 13, 3155–3167.
- Iqbal, H.; Ali, S.; Shahzadi, S. Anti-Inflammatory and Acute Toxicity Study of Organotin (IV) Complexes: A Review. Chem. J. 2016, 6, 59–73.
- Iqbal, M.; Ali, S.; Haider, A.; Khalid, N. Therapeutic Properties of Organotin Complexes with Reference to Their Structural and Environmental Features. Rev. Inorg. Chem. 2017, 37, 51–70.
- Ahmad Shah, S.S.; Ashfaq, M.; Waseem, A.; Ahmed, M.M.; Najam, T.; Shaheen, S.; Rivera, G. Synthesis and Biological Activities of Organotin(IV) Complexes as Antitumoral and Antimicrobial Agents. A Review. Mini Rev. Med. Chem. 2015, 15, 406–426.
- Adokoh, C.K. Therapeutic Potential of Dithiocarbamate Supported Gold Compounds. RSC Adv. 2020, 10, 2975–2988.
- Cattaruzza, L.; Fregona, D.; Mongiat, M.; Ronconi, L.; Fassina, A.; Colombatti, A.; Aldinucci, D. Antitumor Activity of Gold(III)-Dithiocarbamato Derivatives on Prostate Cancer Cells and Xenografts. Int. J. Cancer 2011, 128, 206–215.
- Kamaludin, N.F.; Ismail, N.; Awang, N.; Mohamad, R.; Pim, N.U. Cytotoxicity Evaluation and the Mode of Cell Death of K562 Cells Induced by Organotin (IV) (2-Methoxyethyl) Methyldithiocarbamate Compounds. J. Appl. Pharm. Sci. 2019, 9, 10–15.
- Jakšić, Ž. Mechanisms of Organotin-Induced Apoptosis. In Biochemical and Biological Effects of Organotins; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 149–163.
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593.
- Basu, A.; Krishnamurthy, S. Cellular Responses to Cisplatin-Induced DNA Damage. J. Nucleic Acids 2010, 2010, 201367.
- Yusof, E.N.M.; Latif, M.A.M.; Tahir, M.I.M.; Sakoff, J.A.; Simone, M.I.; Page, A.J.; Veerakumarasivam, A.; Tiekink, E.R.T.; Ravoof, T.B.S.A. O-Vanillin Derived Schiff Bases and Their Organotin(Iv) Compounds: Synthesis, Structural Characterisation, in-Silico Studies and Cytotoxicity. Int. J. Mol. Sci. 2019, 20, 854.
- Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organo-Tin Antitumor Compounds: Their Present Status in Drug Development and Future Perspectives. Inorganica Chim. Acta 2014, 423, 26–37.
- Devi, J.; Pachwania, S. Recent Advancements in DNA Interaction Studies of Organotin(IV) Complexes. Inorg. Chem. Commun. 2018, 91, 44–62.
- Sirajuddin, M.; Ali, S.; Haider, A.; Shah, N.A.; Shah, A.; Khan, M.R. Synthesis, Characterization, Biological Screenings and Interaction with Calf Thymus DNA as Well as Electrochemical Studies of Adducts Formed by Azomethine and Organotin(IV) Chlorides. Polyhedron 2012, 40, 19–31.
- Hussain, S.; Ali, S.; Shahzadi, S.; Tahir, M.N.; Shahid, M. Synthesis, Characterization, Single Crystal XRD and Biological Screenings of Organotin(IV) Derivatives with 4-(2-Hydroxyethyl)Piperazine-1-Carbodithioic Acid. J. Coord. Chem. 2016, 69, 687–703.
- Liu, K.; Yan, H.; Chang, G.; Li, Z.; Niu, M.; Hong, M. Organotin(IV) Complexes Derived from Hydrazone Schiff Base: Synthesis, Crystal Structure, in Vitro Cytotoxicity and DNA/BSA Interactions. Inorganica Chim. Acta 2017, 464, 137–146.
- Shaheen, F.; Sirajuddin, M.; Ali, S.; Zia-ur-Rehman; Dyson, P.J.; Shah, N.A.; Tahir, M.N. Organotin(IV) 4-(BenzoDioxol-5-Ylmethyl)Piperazine-1-Carbodithioates: Synthesis, Characterization and Biological Activities. J. Organomet. Chem. 2018, 856, 13–22.
