Ultraviolet radiation (UVR) tends to damage key cellular machinery. Cells may adapt by developing several defence mechanisms as a response to such damage; otherwise, their destiny is cell death. Since cyanobacteria are primary biotic components and also important biomass producers, any drastic effects caused by UVR may imbalance the entire ecosystem. Cyanobacteria are exposed to UVR in their natural habitats. This exposure can cause oxidative stress which affects cellular morphology and vital processes such as cell growth and differentiation, pigmentation, photosynthesis, nitrogen metabolism, and enzyme activity, as well as alterations in the native structure of biomolecules such as proteins and DNA. The high resilience and several mitigation strategies adopted by a cyanobacterial community in the face of UV stress are attributed to the activation of several photo/dark repair mechanisms, avoidance, scavenging, screening, antioxidant systems, and the biosynthesis of UV photoprotectants, such as mycosporine-like amino acids (MAAs), scytonemin (Scy), carotenoids, and polyamines.
- mycosporine-like amino acids
- photoprotection
- photo repair
- resilience
- scytonemin
1. Introduction
2. Impact of UVR on Cyanobacteria
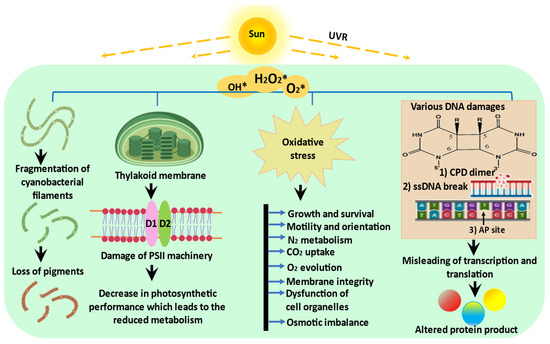
2.1. Photosynthesis
2.2. Growth, Cell Differentiation, and Motility
2.3. Nitrogen Metabolism
2.4. Biomolecules
This entry is adapted from the peer-reviewed paper 10.3390/ijms241512381
References
- Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90.
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.-P.; et al. UVR and cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169.
- Häder, D.-P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 267–285.
- Caldwell, M.M.; Björn, L.O.; Bornman, J.F.; Flint, S.D.; Kulandaivelu, G.; Teramura, A.H.; Tevini, M. Effects of increased solar UVR on terrestrial ecosystems. J. Photochem. Photobiol. B Biol. 1998, 46, 40–52.
- Araújo, R.G.; Alcantar-Rivera, B.; Meléndez-Sánchez, E.R.; Martínez-Prado, M.A.; Sosa-Hernández, J.E.; Iqbal, H.M.; Parra-Saldivar, R.; Martínez-Ruiz, M. Effects of UV and UV-vis irradiation on the production of microalgae and macroalgae: New alternatives to produce photobioprotectors and biomedical compounds. Molecules 2022, 27, 5334.
- Karentz, D.; Cleaver, J.E.; Mitchell, D.L. DNA damage in the Antarctic. Nature 1991, 350, 28.
- Vincent, W.F.; Neale, P.J. Mechanisms of UV damage to aquatic organisms. In The Effects of UV Radiation on Marine Ecosystems; de Mora, S.J., Demers, S., Vernet, M., Eds.; Cambridge Univ. Press: Cambridge, UK, 2000; pp. 149–176.
- Hargreaves, A.; Taiwo, F.A.; Duggan, O.; Kirk, S.H.; Ahmad, S.I. Near-ultraviolet photolysis of b-phenylpyruvic acid generates free radicals and results in DNA damage. J. Photochem. Photobiol. B Biol. 2007, 89, 110–116.
- Gao, K.; Yu, H.; Brown, M.T. Solar PAR and UV radiation affects the physiology and morphology of the cyanobacterium Anabaena sp. PCC 7120. J. Photochem. Photobiol. B Biol. 2007, 89, 117–124.
- Sinha, R.P.; Häder, D.-P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289.
- Kavitha, G.; Kurinjimalar, C.; Thevanathan, R.; Rengasamy, R. Evaluation of Ultraviolet-B radiation induced changes in biochemical response of Arthrospira platensis (Gomont). Int. Res. J. Biol. Sci. 2015, 4, 52–59.
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and UVR-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659.
- Jordan, B.R.; He, J.; Chow, W.S.; Anderson, J.M. Changes in mRNA levels and polypeptide subunits of ribulose 1, 5-bisphosphate carboxylase in response to supplementary ultraviolet-B radiation. Plant Cell Environ. 1992, 15, 91–98.
- Sinha, R.P.; Häder, D.-P. Effects of ultraviolet-B radiation in three rice field cyanobacteria. J. Plant Physiol. 1998, 153, 763–769.
- Campbell, D.; Eriksson, M.J.; Öquist, G.; Gustafsson, P.; Clarke, A.K. The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 364–369.
- Bhandari, R.; Sharma, P.K. High-light-induced changes on photosynthesis, pigments, sugars, lipids and antioxidant enzymes in freshwater (Nostoc spongiaeforme) and marine (Phormidium corium) cyanobacteria. Photochem. Photobiol. 2006, 82, 702–710.
- Singh, S.P.; Rastogi, R.P.; Sinha, R.P.; Häder, D.-P. Photosynthetic performance of Anabaena variabilis PCC 7937 under simulated solar radiation. Photosynthetica 2013, 51, 259–266.
- Gupta, R.; Bhadauriya, P.; Chauhan, V.S.; Bisen, P.S. Impact of UV-B radiation on thylakoid membrane and fatty acid profile of Spirulina platensis. Curr. Microbiol. 2008, 56, 156–161.
- Häder, D.-P. Effects of enhanced solar UVR on aquatic ecosystems. In Biophysics of Photoreceptors and Photomovements in Microorganisms; Lenci, F., Ghetti, F., Colombetti, G., Häder, D.-P., Song, P.-S., Eds.; Springer: New York, NY, USA, 1991; pp. 157–172.
- He, Y.Y.; Häder, D.-P. Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem. Photobiol. Sci. 2002, 1, 729–736.
- Pathak, J.; Singh, P.R.; Häder, D.P.; Sinha, R.P. UV-induced DNA damage and repair: A cyanobacterial perspective. Plant Gene 2019, 19, 100194.
- Döhler, G.; Biermann, I.; Zink, J. Impact of UV-B radiation on photosynthetic assimilation of IT-bicarbonate and inorganic ‘SN-compounds by cyanobacteria. Z. Naturforsch. 1986, 41, 426–432.
- Häder, D.-P.; Watanabe, M.; Furuya, M. Inhibition of motility in the cyanobacterium Phormidium uncinatum by solar and monochromatic UV irradiation. Plant Cell Physiol. 1986, 27, 887–894.
- Newton, J.W.; Tyler, D.D.; Slodki, M.E. Effect of ultraviolet-B (280–320 nm) radiation on blue-green algae (Cyanobacteria), possible biological indicators of stratospheric ozone depletion. Appl. Environ. Microbiol. 1979, 37, 1134–1141.
- Richa, S.R.; Sinha, P. UV-mediated stress and its mitigation in cyanobacteria. Int. J. Plant Anim. Environ. Sci. 2011, 1, 155–166.
- Sinha, R.P.; Singh, N.; Kumar, A.; Kumar, H.D.; Häder, D.-P. Effects of UV irradiation on certain physiological and biochemical processes in cyanobacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 107–113.
- Kumar, A.; Tyagi, M.B.; Jha, P.N.; Srinivas, G.; Singh, A. Inactivation of cyanobacterial nitrogenase after exposure to ultraviolet-B radiation. Curr. Microbiol. 2003, 46, 0380–0384.
- Tyagi, R.; Srinivas, G.; Vyas, D.; Kumar, A.; Kumar, H.D. Differential effect of ultraviolet-B radiation on certain metabolic processes in a chromatically adapting Nostoc. Photochem. Photobiol. 1992, 55, 401–407.
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89.
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Effects of PAR and UV radiation on the structural and functional integrity of phycocyanin, phycoerythrin and allophycocyanin isolated from the marine cyanobacterium Lyngbya sp. A09DM. Photochem. Photobiol. 2015, 91, 837–844.
- Wu, H.; Gao, K.; Villafañe, V.E.; Watanabe, T.; Helbling, E.W. Effects of solar UVR on morphology and photosynthesis of filamentous cyanobacterium Arthrospira platensis. Appl. Environ. Microbiol. 2005, 71, 5004–5013.
- Bhandari, R.; Sharma, P.K. Effect of UV-B and high visual radiation on photosynthesis in freshwater (Nostoc spongiaeforme) and marine (Phormidium corium) cyanobacteria. Indian J. Biochem. Biophys. 2007, 44, 231–239.
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980.
- Xie, Z.; Wang, Y.; Liu, Y.; Liu, Y. Ultraviolet-B exposure induces photo-oxidative damage and subsequent repair strategies in a desert cyanobacterium Microcoleus vaginatus Gom. Eur. J. Soil Biol. 2009, 45, 377–382.
- Sinha, R.P.; Dautz, M.; Häder, D.-P. A simple and efficient method for the quantitative analysis of thymine dimers in cyanobacteria, phytoplankton and macroalgae. Acta Protozool. 2001, 40, 187–195.
- Mosca, C.; Rothschild, L.J.; Napoli, A.; Ferré, F.; Pietrosanto, M.; Fagliarone, C.; Billi, D. Over-expression of UV-damage DNA repair genes and ribonucleic acid persistence contribute to the resilience of dried biofilms of the desert cyanobacetrium Chroococcidiopsis exposed to Mars-like UV flux and long-term desiccation. Front. Microbiol. 2019, 10, 2312.
