Hypertension (HTN) is defined as a pathological disorder that is characterized by elevated blood pressure (BP), i.e., the systolic around 140–150 or above [
1]. The World Health Organization (WHO) has identified HTN as one of the leading causes of mortality and morbidity globally, accounting for nearly 9 million deaths yearly [
2]. Substantial scientific studies have linked high blood pressure (BP) to various disorders, such as cardiovascular disorders and heart failure [
3]. Hypertension is also considered a significant risk factor for the development of angina pectoris, chronic kidney disease (CKD), diabetes miletus (DM), and atrial fibrillation [
4,
5]. Hypertension and diabetes are considered to often coexist together. The risk of developing diabetes, mostly type 2, is higher in patients with hypertension than in healthy people. Following that, several clinical trials and epidemiological studies revealed a link between antihypertensive drugs (e.g., thiazide diuretics or beta-blockers) and the development of diabetes (type 2). Some reported studies have found that beta-blockers, in particular, appear to enhance the incidence of diabetes in hypertensive patients [
6]. Apart from this, the elevated risk linked to high BP can mostly be considerably reduced with antihypertensive therapy, which reduces both associated target organ damage and BP [
7,
8]. The first-line treatment of HTN could be chosen from the following classes: angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACE inhibitors), calcium channel blockers (CCBs), and thiazide-type diuretics. Each antihypertensive class lowers the risk of cardiovascular events [
9]. It is challenging for hypertensive patients to follow their therapeutic regimens using traditional dosage forms, including tablets, capsules, and injections [
10]. The pediatric population suffering from hypertension has trouble swallowing medications and is allergic to needles [
5]. Most antihypertensive drugs are given in the form of tablets; nevertheless, tablets have several disadvantages, including gastrointestinal discomfort, drug degradation in the stomach, irregular absorption, and pre-systemic drug metabolism, eventually leading to lower bioavailability [
10,
11]. Such issues can be solved to some extent by administering antihypertensive drugs via transdermal microneedle (MN)-based drug delivery systems [
12]. The restrictions of the oral and injectable routes are bypassed with the MN-based transdermal drug delivery system, as the needles are non-invasive, low-cost, and easy to use [
13,
14,
15,
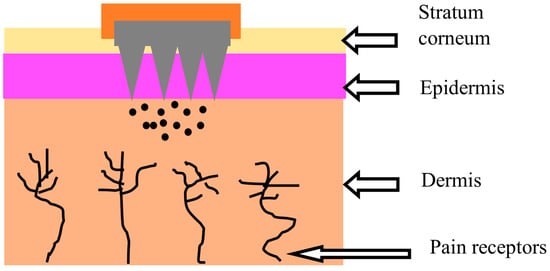
16]. They are micron-sized needles that are less than 1000 µm in size and can penetrate the principal barriers of the skin that inhibit drug molecules’ transport through the stratum corneum without causing pain (
Figure 1) [
15,
17]. MN systems can provide precise drug localization with reduced dosing frequency and improve patient compliance through the convenience of administration and better biodistribution with efficacy [
18]. A significant problem for the therapeutic application of antihypertensive medications is their limited aqueous solubility.
2. Fabrication and Current Status of Microneedles
2.1. Microneedle Design and Fabrication
When designing MNs for skin penetration, there are a few important factors to consider: (a) physical characteristics, such as hollow, solid, side-opened, beveled, and conical tipped; (b) material choice; (c) geometric characteristics, such as diameter, length, shape, and tip size; (d) array layout; (e) fabrication feasibility [
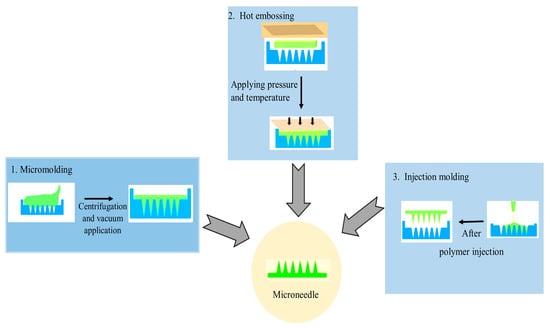
113]. Some MN fabrication technologies include wet chemical etching, injection molding, laser drilling, reactive ion etching, hot embossing, drawing lithography, and lithography with electroforming (
Figure 2). To date, silicon deep reactive ion etching (DRIE), micromolding, and photolithography are the most extensively used production techniques for the fabrication of microneedles [
113,
114]. Microelectromechanical systems are commonly used to fabricate microneedles. The basic procedure for making MNs can be broken down into three steps: (a) deposition, making a deposit, (b) patterning, creating patterns, and (c) etching or engraving [
115]. There are various ways to manufacture microneedle devices, including surface/bulk micromachining, injection molding, reactive ion etching, isotropic chemical etching, and so on [
115].
Figure 2. Various casting and molding techniques are used for the preparation of MNs.
2.1.1. Silicon MN Fabrication
Silicon MNs have been the most prevalent form of MNs, manufactured using microfabrication techniques that require complex multistep processes and expensive tools explicitly made for the microelectronics industry. Wet and dry etching, which are subtractive methods, are the most commonly used methods for fabrication [
113]. Before creating the MNs from the front side of the silicon wafer, the microneedle channels were first etched from the backside of the wafer using a combination of isotropic and anisotropic etching [
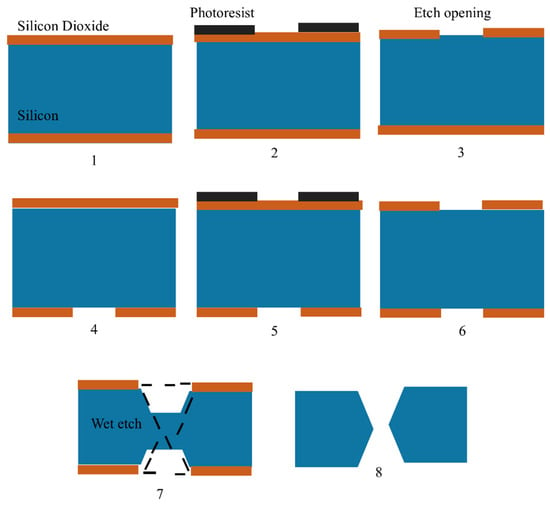
120]. The fabrication of silicon MNs is shown in
Figure 3. Silicon MNs are fabricated using silicon wafers with double-sided polish and a thickness of 300 µm. Thermal silicon dioxide is generated on the wafers’ front and back. Fabrication of silicon MNs consists of seven steps, which are as follows: (1) A 300 µm wafer is coated with 2.5 µm of silicon dioxide via chemical vapor deposition, (2) the silicon-dioxide hard mask is then etched with the device pattern using the inductively linked plasma etching technique, (3) this is followed by the wafer flipping and patterned utilizing alignment marks on the backside by photolithography, (4) inductively linked plasma etching is used on the device pattern etched into the silicon dioxide hard mark, (5) the entire wafer is then immersed in a 44% potassium hydroxide solution to etch it, (6) the etching process takes five hours, (7) finally, the equipment is taken out of the potassium hydroxide solution [
121]. (
Figure 3).
Figure 3. Figure showing steps for in-plane silicon microneedle fabrication as shown by Howells, et al., 2022. Silicon wafers are represented in blue, silicon dioxide is shown in brown and photoresist is shown in black.
3.1.2. Polymer MN Fabrication
Polymer materials are gaining popularity because of their outstanding mechanical qualities, biocompatibility, and biodegradability. Polymer microneedles also have a lower fabrication cost than silicon microneedles. Photolithography, micromolding, and micromachining are increasingly becoming the preferred production processes for developing polymer microneedles [
113,
114]. Photolithography is the process of chemically modifying a liquid material and then exposing it to short-wavelength light to polymerize it. The resist is sprayed onto the substrate surface for patterning and later exposed to light (typically UV light) using a projection stepper, followed by wet development to form a resist pattern. This procedure requires a photosensitive material like polymethylmethacrylate for fabrication [
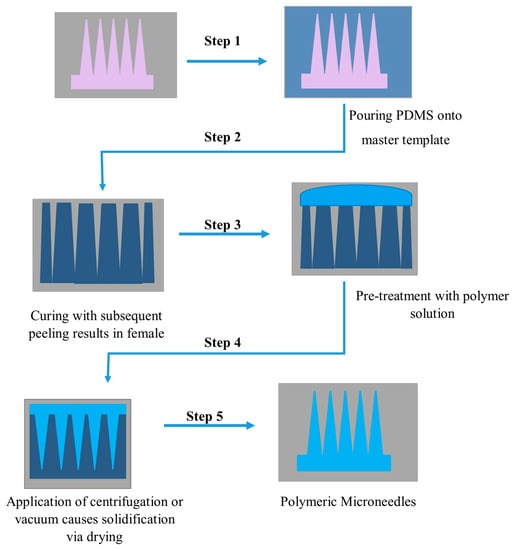
113]. The fabrication of MNs by micromolding is shown below in
Figure 4 [
113]:
Figure 4. Schematic illustration of polymeric microneedle fabrication via polydimethylsiloxane PDMS micro molding.
2.2. Diverse Application of MNs in Advanced Drug Delivery
When MNs are designed to fit the qualities of drugs, such as their polarity and pharmacological characteristics, they are regarded as an appropriate method for delivering various drug moieties via continuous transdermal delivery. Typically, drugs are inserted into the microneedle matrix or deposited onto the surface of the microneedle tips. The drugs can sometimes be preloaded into nanoparticles encased in the microneedle matrix to regulate the medication release profile [
123]. The latest advancements in drug delivery via MNs include small molecule delivery, insulin delivery, cosmeceuticals, and cancer treatment [
124]. Drugs possessing molecular weights below 500 Da can penetrate the skin passively, but the penetrated amounts are insufficient to produce therapeutically effective doses. As a result, polymeric MNs have been used to improve the transdermal delivery of drugs with small molecular sizes [
122,
125].
2.3. Mechanism, Pharmacokinetics, and Insertion Behavior of MNs
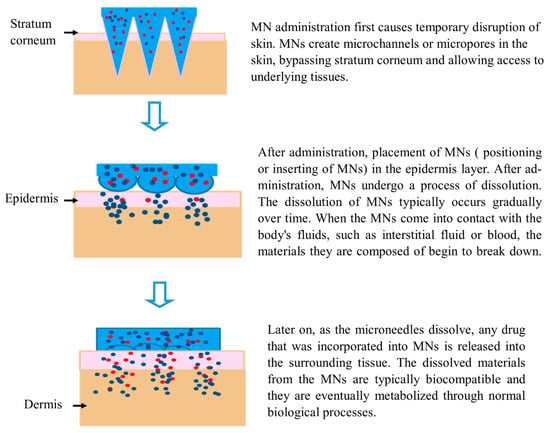
Hundreds of MNs, each less than 1 mm long, are arranged in an MN array to deliver medication to the skin. An MN patch is developed when an MN array is connected to an adhesive backing to help its adhesion to the skin [
126]. The drug is deposited into the dermis layer of the skin, where it can easily reach its intended target site as part of the drug delivery process, resulting in temporary mechanical disruption of the skin (
Figure 5). Additionally, MNs provide microscale drug delivery channels while bypassing still-functioning blood vessels and nerve terminals in the epidermis and dermis. As a result, drug delivery efficiency is improved, and greater doses and medicines with larger molecular sizes can be administered without difficulty [
127]. For example, biodegradable polymers have been utilized to fabricate polymeric MN-containing drugs inside a polymer matrix. These MNs puncture the skin; the polymers disintegrate, releasing the medications into the bloodstream and causing a therapeutic reaction at the site of action [
79].
Figure 5. Mechanism of action of MNs. Blue color represents a fast drug releasing mechanism, while red color indicates a slow-release mechanism.
Following MN application, pharmacokinetics explains how the body reacts to a therapeutic moiety and how it flows through, out of, and into the body (including metabolic changes, absorption, and distribution mechanisms) [
124]. The pharmacokinetic profile of MNs can be influenced by several factors, such as the rate at which the polymer matrix dissolves, the total drug dose injected into the MN, and the enzymatic degradation of the drug into the skin [
97]. Lee’s research team employed a dissolving polymeric MN composed of carboxymethylcellulose and gelatin to deliver insulin to diabetic rats. They found that the pharmacokinetic parameter area under the curve (AUC) value increased following the MN application. Polymeric MNs for insulin administration showed adequate pharmacokinetics compared to typical hypodermic injections [
128]. These findings suggest that MNs could be extremely effective transdermal drug delivery systems. MNs offer much potential for the quick, painless, and convenient administration of medications by fulfilling pharmacokinetic requirements.
The insertion behavior of MNs into the skin can also be influenced by the structure and mechanical properties of the skin, as determined by Kong and Wu [
129]. Factors, such as the effects of the MN length on effective drug delivery and associated pain, must be considered while designing MNs [
130]. The lowest insertion force is always expected because it naturally signifies less pain and invasiveness [
131].
2.4. Advantages of MNs for Drug Delivery, Patient Monitoring, Diagnostics, and Vaccine Delivery
MNs have potential advantages over conventional hypodermic needles for drug and vaccine delivery. MNs are designed with unique dimensions to avoid stimulating nerves and causing patient discomfort. MNs have the potential to be administered without clinical expertise, as they are in the form of affordable disposable patches to improve the pharmacokinetic profile of therapeutic component delivery. For example, disposable MN patches could reduce the transmission of HIV by encouraging the self-administration of tests and treatments [
113]. Some other advantages of MN-based drug delivery are: faster healing at the injection site than a hypodermic needle, decreased microbial penetration, the MN punctures only the epidermis, specific skin areas can be targeted for desired drug delivery, dose reduction, and the drug delivery rate can be controlled more effectively by this drug delivery system [
132].
Nowadays, MNs have been employed as a diagnostic aid in managing diseases. The traditional methods for blood withdrawal are characterized by discomfort and fear among patients; this can be avoided by employing MNs through their painless biofluid withdrawal method. With their ease of usage, MNs represent a unique tool for detecting a wide range of biomarkers from skin interstitial fluid, including small molecule metabolites, nucleic acids, proteins, and even cells. Recent studies revealed the status of diseases (cancer, diabetes, arthritis, etc.) by analyzing blood and tissue using hollow MNs or quantum dots [
133].
MNs have been widely investigated in the past years to enhance transdermal drug delivery. Recently, researchers have realized the potential of MNs for enhancing patient monitoring. Several methods of patient monitoring with MNs have been proposed, including using solid arrays for pretreatment before fluid collection, hollow microneedle arrays for fluid collection and subsequent off-site analysis, and integrated options, eliminating the need for fluid removal. Regardless of the strategy, the MN device must successfully and repeatedly penetrate without fracture and provide precise measurements of the target analytes to be a useful alternative to existing practices. When looking into MN platforms for minimally invasive patient monitoring, there are many factors to consider, and many different strategies have been taken into account [
134].
The use of MNs in vaccination is particularly appealing since it provides the anticipated benefits of simplifying vaccine administration, enhancing patient compliance, and permitting vaccine targeting to the skin. It is well recognized that administering vaccines through the skin has immunologic benefits over doing so by intramuscular injection. Still, there have not been any straightforward, dependable techniques for doing so. This constraint can be overcome by using MNs, including hollow MNs for intradermal injection and solid MN patches. The use of MNs for vaccine delivery has received the most research attention due to these opportunities [
85].
There are different routes for drug delivery, which include oral, intravenous, transdermal patches, etc. The oral route is the most traditional and convenient for patients, with acceptable ease of administration but limited bioavailability. The oral route adversely affects long-term medications because it affects crucial organs, including the liver and kidneys [
100]. However, a transdermal patch requires the drug to cross the stratum corneum barrier, thus showing less bioavailability. The transdermal patch can improve drug permeation by adding a permeation enhancer, but up to a minimal extent. The hypodermic needle goes deep into the dermis, where pain receptors are present. As a result, it can deliver 90–100% of the loaded drug, but because it is painful, it results in poor patient compliance. MNs bypass the stratum corneum barrier and deliver the drug directly into the epidermis and dermis layers, delivering 100% of the loaded drug without causing pain [
79].
2.5. MN-Mediated Antihypertensive Agents and Some Reported Nanoparticle-Based Delivery Systems
The link between cardiovascular disease and high blood pressure is widely established in the scientific literature. Hypertension can lead to kidney failure and coronary artery disease if left untreated. Hypertension is still a life-threatening medical issue, despite recent breakthroughs in hypertension research and therapies [
1]. As a result, it is imperative to develop and test innovative hypertensive medicines to enhance patients’ long-term clinical care and results. Several hypertension animal models have been developed recently to facilitate in vivo testing of both treatment methods and medication efficacy [
135].
Recently, MNs have been used as an alternative drug delivery system to deliver antihypertensive drugs across the skin barrier painlessly. MN-based drug delivery reduces the risk of adverse effects associated with oral antihypertensive medications. Additionally, MNs can be used to deliver sustained-release formulations of antihypertensive drugs, which can help improve patient compliance and reduce dosing frequency. Overall, MN-based drug delivery of antihypertensive drugs has the potential to be a safe and effective treatment option for patients with hypertension. Recently, much research has been going on to develop and optimize the delivery system for different antihypertensive drugs [
24,
136]. Some research utilizing MN-based drug delivery of antihypertensive drugs is discussed here.
An antihypertensive dissolving MN was developed utilizing concurrent medicine, e.g., sodium nitroprusside (SNP) in combination with sodium thiosulfate (ST). Dissolvable MNs were fabricated by centrifugal casting using SNPs and ST. SNPs were stably packaged into microneedles and rapidly delivered into the systemic circulation using this approach. Antihypertensive microneedle treatment (aH-MN) reduced blood pressure quickly and significantly. It met the clinical standards for hypertensive emergency blood pressure treatment. Concurrent delivery of ST successfully decreased the negative effects (e.g., organ damage) caused by SNP ingestion. This research demonstrated an effective and user-friendly biodegradable patch for the controllable delivery of drugs in antihypertensive therapy [
23,
137]. Ahad et al. formulated eprosartan mesylate-loaded transferosome gel to study skin permeation. The pharmacodynamic study showed better management of hypertension after the application of transferosome gel as compared to an oral formulation. An MN roller, e.g., a dermaroller, was utilized on rats’ skin to increase the permeation enhancement of the drug. The transdermal flux of the eprosartan mesylate from transferosome gel across rat skin improved when pretreated with an MN [
138].
2.5.1. Calcium Channel Blockers (CCB)
Calcium channel blockers are generally well absorbed following oral administration. However, all calcium channel blockers undergo significant first-pass metabolism, dramatically lowering oral bioavailability. Traditional dosage forms have significant drawbacks, including hepatic first-pass metabolism, a high incidence of side effects due to varied absorption profiles, a higher frequency of administration, and poor patient compliance. Attempts have been undertaken to develop novel drug-delivery systems for various calcium channel blockers, including microneedle-based delivery systems, to overcome conventional drug delivery’s disadvantages [
31]. In 2014, Kaur et al. fabricated MN rollers and solid stainless steel MNs using amlodipine besylate and verapamil hydrochloride. Passive penetration of verapamil and amlodipine across the skin was observed to be very low. To improve the percutaneous penetration of these antihypertensive drugs, the effect of the fabricated MNs was observed. It was mentioned that the percutaneous flux of amlodipine besylate after using stainless steel MNs was significantly enhanced.
Moreover, the transcutaneous flux of verapamil across MN-roller-treated porcine skin was also increased. The author has claimed that stainless steel solid MNs and MN rollers increased percutaneous penetration of verapamil hydrochloride and amlodipine besylate significantly [
139]. Another study was conducted to investigate the effect of MN parameters like shape, length, density, and type on the enhancement of transdermal permeation using an antihypertensive drug, e.g., amlodipine. The study showed that MN application enhanced amlodipine transdermal permeation, and MN geometrical parameters play a significant role in such permeation enhancement [
140]. Sardesai et al. formulated a bio-responsive MN with an advanced drug delivery system by incorporating nifedipine, a cardiodepressant, and diltiazem, a vasodilator, for effective synergism to treat hypertension. The study revealed that formulated MN systems co-delivering antihypertensive drugs significantly and substantially lowered blood pressure compared to conventional drug delivery systems [
141]. Another study was conducted in which MN rollers were developed for the transdermal permeation of two antihypertensive drugs, e.g., diltiazem hydrochloride and perindopril erbumine. This study showed increased transdermal permeation using an antihypertensive drug-loaded MN roller [
1]. Alkilani et al. conducted a study to develop and evaluate a transdermal delivery system of amlodipine besylate loaded with biodegradable polymeric nanoparticles for sustained drug delivery. A study claimed transdermal delivery of amlodipine besylate in a controlled manner by incorporating biodegradable polymeric nanoparticles in hydrogel MN [
142]. Kolli et al. used solid maltose MNs made by micromolding techniques to instantly deliver nicardipine hydrochloride across rat skin. MNs create microchannels in the skin, which have been shown to improve the transdermal delivery of nicardipine hydrochloride [
108].
2.5.2. Angiotensin-Converting Enzyme Inhibitors (ACE)
Angiotensin-converting enzyme inhibitors have been used for many years to manage heart failure and hypertension. ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, lowering blood pressure by reducing blood vessel tension and blood volume. ACE inhibitors are currently administered either intravenously or orally. Many researchers have investigated MN-based drug delivery as an advanced alternative approach for ACE inhibitor delivery over the years [
143]. Stainless steel MN arrays and rollers were developed to study the transcutaneous flux of captopril and metoprolol tartrate across the skin. There was a significant enhancement in the transdermal flux and permeation across the skin for captopril and metoprolol tartrate following MN arrays and rollers [
144].
2.5.3. Angiotensin II Receptor Antagonists (ARB)
Angiotensin II receptor antagonists are a group of pharmaceuticals that modulate the renin–angiotensin–aldosterone system. ARBs may be used alone or in combination with other medications, frequently diuretics. When patients cannot tolerate ACE medication for their hypertension, ARBs are usually preferred. ARBs show extensive first-pass metabolism and poor availability when given orally. MN-based drug delivery can avoid poor bioavailability and other limitations of ARB. Several studies have been investigated to develop and study the effect of MN-based delivery of ARBs [
52]. Huang et al. fabricated an MN patch system composed of dissolving gelatin and starch and loaded with losartan. The dissolving MN patches effectively penetrated the stratum corneum and exhibited strong mechanical strength.
The study showed that the developed MN patch system increased the drug delivery efficiency of losartan upon dissolution of gelatin and starch [
145]. Pineda-Álvarez et al. formulated biodegradable polymeric MN arrays loaded with losartan potassium nanoparticles as a novel pharmaceutical drug delivery intended to be used for blood pressure control. The study revealed that the optimal formulation of the MN array with nanoparticles constitutes the most appropriate option for the transdermal delivery of losartan [
146]. Enggi et al. developed valsartan transdermal gel to overcome valsartan’s poor absorption and low bioavailability. Moreover, solid MNs were combined with transdermal gel to improve the percutaneous permeation of valsartan across the stratum corneum. The study revealed that using MNs significantly enhanced valsartan permeation across the skin and significantly impacted the treatment of hypertension [
147]. Another research study was conducted to determine the release of valsartan from transdermal patches and the effect of solid MN on its permeation. The transdermal patch consists of polyethylene glycol 400 as a permeation enhancer. The study revealed that incorporating polyethene glycol enhanced valsartan’s permeation across the skin. Notably, a combination of transdermal patches and MNs significantly improved the permeation of valsartan [
148]. Likewise, Nirmayanti et al. developed a thermosensitive hydrogel for sustained transdermal delivery of valsartan, further enhanced by solid MNs.
The formulation, containing hydrogel and solid MNs, increased the permeation of valsartan. The transdermal delivery of valsartan using this combinational approach improved the bioavailability of the drug as compared to oral delivery [
149]. Moreover, another study described the simultaneous delivery of a combination of three drugs (aspirin, lisinopril dihydrate, and atorvastatin calcium trihydrate) using a dissolving polymeric MN system. Studies showed that it is possible to deliver a combination of drugs from a single-dissolving MN array [
150]. Almazan et al. developed a losartan potassium patch using MN for the treatment of hypertension and its complications, where components of the matrix system permit a controlled drug release. This matrix system increases the bioavailability of losartan and avoids multiple doses. The authors claimed that multiple doses, dose-associated side effects, and gastric irritations could be avoided, making the matrix system more convenient for patients [
151].