In the last few decades, type 2 diabetes mellitus (T2DM) has dramatically increased in prevalence worldwide, resulting in significant burdens on patients suffering from this condition and healthcare systems [
1]. Of note, the rising prevalence of this disease is associated with the development of a wide range of complications, including retinopathy, nephropathy, neuropathy, and cardiovascular disease [
1,
2]. These complications often affect the quality of life of patients with T2DM, including their physical and psychological functioning [
3]. Although some of these comorbidities have a well-known impact on the quality of life [
4,
5], others have received less attention [
6].
Mounting evidence reveals that bone fragility is common in T2DM [
7]. Several studies have shown that T2DM constitutes an independent risk factor for osteoporotic fractures, presenting a particularly strong association with hip fractures [
8,
9,
10,
11]. Indeed, a number of meta-analyses have confirmed that T2DM is associated with an increased risk of incident hip, vertebral, and non-vertebral fractures [
12,
13,
14]. Since T2DM has a strong relationship with hip fractures that need replacement surgery using total hip arthroplasty, new techniques have been developed in this field [
15,
16]. Importantly, increases in the incidence of fractures lead to greater costs and healthcare resource utilization in this population [
17]. Moreover, fractures are associated with functional impairment and reduction of health-related quality of life [
18,
19]. Given the important health and socioeconomic impact of skeletal fragility and fractures, individuals with T2DM, especially those with major diabetes-related determinants and other conventional risk factors for osteoporosis, should be assessed for the presence of bone fragility and their fracture risk [
20]. However, traditional imaging techniques and fracture risk assessment tools may not be accurate for this purpose in patients with T2DM [
21].
2. Determinants of Skeletal Fragility and Increased Risk of Fracture in T2DM
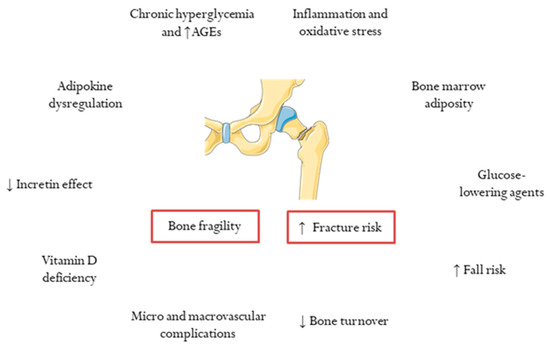
Several determinants have been identified in the pathogenesis of bone fragility and increased fracture risk in subjects with T2DM [
23] (
Figure 1). Notably, a longer duration of T2DM was reported to be an independent risk factor for major osteoporotic fractures in women aged ≥40 and with ≥10 years of diabetes duration [
24], and a recent meta-analysis showed a greater increase in the risk of both hip and non-vertebral fractures in subjects with longer diabetes duration [
13]. Besides this, poor glycemic control is closely linked to fracture risk, as several large-scale population-based cohort studies have demonstrated [
25,
26,
27]. In this regard, the generation of advanced glycation end-products (AGEs) resulting from chronic exposure to hyperglycemia is one of the key mechanisms in the pathophysiology of bone fragility in T2DM [
23]. As such, non-enzymatic glycosylation of collagen leads to the formation of collagen-AGEs, which are involved in the development of impaired bone mineralization and quality through different alterations of the extracellular matrix, a reduction of alkaline phosphatase activity in osteoblasts, and an overactivation of the receptor for AGEs (the latter associated with the release of pro-inflammatory cytokines and reactive oxygen species—ROS—by osteoclasts) [
23,
28]. On the other hand, it is also postulated that the main event related to bone fragility in T2DM is an overall inhibition of bone cells function and decreased bone turnover [
23,
29]. This effect may be driven in part by insulin resistance [
30].
Figure 1. Determinants of bone fragility and increased fracture risk in type 2 diabetes. AGEs, advanced glycation end products.
In addition to chronic hyperglycemia and AGE formation, other mechanisms play a role in bone fragility in T2DM, as previously reviewed [
7,
23,
31]. Among them, a pro-inflammatory state and oxidative stress, along with adipokine dysregulation and marrow adiposity, have a strong influence on bone metabolism [
7,
31]. Loss of incretin effect has also been implicated in the pathogenesis of skeletal fragility in T2DM [
31,
32]. Microvascular disease and impaired vascular bone intercommunication determine alterations of bone quality and microarchitecture [
7,
31]. Ischemic heart disease has also been reported to be associated with an increased risk of vertebral fractures in T2DM [
33]. Vitamin D deficiency, commonly found in patients with T2DM, could play a role in both T2DM development and bone fragility [
34]. Pathological changes in gut microbiota composition in T2DM may also trigger bone alterations in this population [
35].
Further to this, glucose-lowering agents may also be crucial contributors to the reported associations between T2DM and bone fragility [
36,
37]. The potential benefits of some drugs for bone density and fracture risk (i.e., metformin, glucagon-like peptide 1 receptors agonists and dipeptidyl peptidase-4 inhibitors) [
38,
39,
40] remain to be confirmed in specifically designed studies. Conversely, the long-term use of thiazolidinediones has been independently associated with fracture risk [
41], and sodium-glucose cotransporter-2 inhibitors could also have this effect [
42,
43]. Remarkably, both insulin and sulfonylureas significantly increase fall-related fractures due to episodes of hypoglycemia [
44]. In this vein, other prevalent factors in T2DM (i.e., visual impairment, peripheral neuropathy, autonomic dysfunction/postural hypotension, foot ulcers/amputation, and sarcopenia) also lead to an increased risk of fall-related fractures [
31,
45].
3. Bone Density and Fracture Risk Prediction in T2DM
Despite skeletal fragility and fracture risk being greater in subjects with T2DM, this condition is usually associated with normal or even increased BMD measured by DXA [
46]. Thus, women with T2DM in the Women’s Health Initiative Observational Study presented higher hip and spine BMD scores compared to those without T2DM [
47]. Similarly, in a cross-sectional study including two Swedish cohorts, both men and women exhibited a progressively higher hip BMD according to normal fasting plasma glucose/impaired fasting plasma glucose/T2DM subgroups [
48]. In the prospective population-based cohort from the Rotterdam Study, inadequate glycemic control was associated with both higher BMD and increased fracture risk in participants with T2DM [
27]. Furthermore, a meta-analysis of 15 observational studies (3473 subjects with T2DM and 19,139 healthy controls) showed that participants with T2DM had significantly higher BMD at the femoral neck, hip, and spine [
49].
It is noteworthy that these results contrast with those reported by studies assessing BMD in type 1 diabetes mellitus (T1DM), in which BMD is generally low [
50]. Although the mechanisms involved in the association between T2DM and normal/high BMD are not fully understood, some data suggest that these findings might be related to chronic hyperinsulinemia and insulin resistance [
51], as well as the effect of some adipokines, such as leptin, on bone metabolism [
52]. Excess weight/obesity, which are often encountered in patients with T2DM, could also play a role in increased BMD, although some studies have reported that this relationship remains after adjusting for the body mass index (BMI) [
49]. Since T2DM is associated with increased fracture risk, regardless of whether there is a normal/high BMD, a fact known as “the diabetic paradox of bone fragility” [
53], the diagnosis of osteoporosis based on BMD measured by DXA, should be cautiously considered [
21].
On the other hand, the Fracture Risk Assessment Tool (FRAX), which is widely used to estimate 10-year absolute fracture risk, has been demonstrated to underestimate the risk for both hip and major osteoporotic fractures in patients with T2DM [
54]. These results are influenced, in part, by the higher BMD observed in patients with T2DM [
49]. Indeed, contrary to T1DM, T2DM is not included in the FRAX tool as a secondary cause of osteoporosis [
55]. In this regard, some authors have proposed a correction factor with the use of glycated hemoglobin in order to improve the predictive ability of this algorithm for fracture risk [
56]. Recently, adjustment of FRAX for T2DM has been suggested in order to create a useful alternative [
57,
58], although further research is warranted to confirm these results. Alternatively, certain methods (i.e., inputting rheumatoid arthritis, adjusting FRAX by TBS, reducing the femoral T-score by 0.5, and increasing the age by 10 years) have been proposed to improve the performance of FRAX in T2DM, although no single method appears to be optimal in all settings [
59]. In light of the above, new approaches to the evaluation of bone fragility in patients with T2DM are needed.
4. Bone Microstructure in T2DM
As previously discussed, patients with T2DM have normal or elevated BMD; however, bone microarchitecture alterations may be present in this group, resulting in an increased fracture risk [
60]. In this context, the trabecular bone score, high-resolution peripheral quantitative computed tomography, and microindentation are useful techniques for the evaluation of the bone microstructure in T2DM.
5. Bone Quality in T2DM: The Role of Biomarkers of Bone Fragility
In addition to bone mineralization and microarchitecture, skeletal material properties are also influenced by bone turnover and the quality of collagen, which may be affected by the accumulation of AGEs, leading to the alteration of collagen crosslinks and function as discussed in previous sections [
23]. In this regard, it has been stated that bone turnover is decreased in T2DM, which results in reduced serum levels of bone remodeling markers [
23,
96,
97,
98]. However, it remains unknown whether these biochemical markers may be helpful for the diagnosis of bone fragility or the prediction of fracture risk in patients with T2DM. On the one hand, decreased circulating levels of parathyroid hormone (PTH) along with osteocalcin were shown to be associated with a higher risk of vertebral fracture in postmenopausal women with T2DM [
99]. On the contrary, in a recent study, Napoli et al. showed that serum bone turnover markers (terminal telopeptide of type 1 collagen-CTX, osteocalcin, and procollagen type 1 N-terminal propeptide-P1NP) were not able to predict fracture risk in T2DM [
100].
On the other hand, AGES related to collagen, such as pentosidine and N-carboxymethyl lysine (CML), are increased in bone biopsy specimens from subjects with T2DM [
60,
101,
102]. Therefore, circulating/urinary levels of these AGEs may become attractive surrogate markers of bone quality in subjects with T2DM. Besides this, other novel biomarkers could play a role in the evaluation of bone fragility in T2DM.