Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
The outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the deadliest virus since the 1918 influenza virus, has posed a serious threat to global health security. Tremendous research efforts have been undertaken, aiming at controlling and/or treating SARS-CoV-2 infection. The exploration of non-toxic and cost-effective dietary components, such as epigallocatechin 3-gallate and myricetin, for health improvement and disease treatment has recently attracted substantial research attention.
- COVID-19
- SARS-CoV-2
- natural dietary flavonoids
- EGCG
- myricetin
1. Introduction
To date, several small-molecule antivirals (remdesivir, ritonavir-boosted nirmatrelvir, and molnupiravir), vaccines, and monoclonal antibodies have been approved or authorized by the Food and Drug Administration (FDA) of the United States of America for the treatment of COVID-19 [5,6,7,8]. Although the pandemic appears to be on a downward trend, the potential emergence of new SARS-CoV-2 variants still represents a threat to humans, given their intrinsic transmissibility, immune escape, virulence, and susceptibility to available treatments [9,10,11]. Taking virulence as an example, if it is assumed that the mortality rate among SARS-CoV-2-infected individuals is similar to that for seasonal influenza, we can expect the annual burden of future influenza to be twice that of previous influenza. The additional burden associated with “long COVID” (e.g., respiratory symptoms and cognitive dysfunction) may also be non-negligible [12,13]. Accordingly, an inexpensive, convenient, and rapidly up-scalable response model is required to address future coronavirus pandemics.
Natural products (including herbal medicine) play an irreplaceable role in the treatment of SARS-CoV-2 infection [14,15]. Increasing evidence supports that many functional foods and nutraceuticals have potential for use in the prevention and treatment of viral infections [16]. In recent years, flavonoids have attracted much attention from pharmaceutical chemists and organic chemists due to their efficiency and low toxicity for health improvement and disease treatment. Their active components, such as epigallocatechin 3-gallate (EGCG) and myricetin (Figure 1), have drawn considerable attention as potential agents for COVID-19 treatment owing to their multitargeting potential (SARS-CoV-2 Mpro, angiotensin-converting enzyme 2 [ACE2, the primary target of SARS-CoV-2 in host cells], and RNA-dependent RNA polymerase [RdRp, an essential enzyme in RNA viruses, which is a key player driving the viral replication and transcription machinery], among other targets), broad-spectrum activities, and low toxicity [17,18].
2. Epigallocatechin 3-Gallate—A Green Tea-Derived, Multitargeting, Anti-SARS-CoV-2 Therapeutic Candidate
Epigallocatechin 3-gallate (EGCG), a nutritional supplement with promising health-beneficial effects isolated from green tea (Camellia sinensis) (Figure 1a), has long been investigated for its potential as supplementation therapy for the prevention of numerous disorders, including cancer [19] and cardiovascular [20], metabolic [21], neurodegenerative [22], and infectious diseases [23]. For instance, Polyphenon E®, comprising >65% EGCG, is a standardized preparation of green tea catechins approved by the US FDA in 2006 for the treatment of external genital and perianal warts [24]. Polyphenon E has an excellent safety and tolerability profile, an essential characteristic allowing for the extensive use of EGCG [24]. The green tea catechin palmitate (comprising 50% EGCG), an oil-soluble green tea extract, was approved by the US FDA in 2019 as a safe dietary ingredient [25]. In vitro, EGCG has highly promising broad-spectrum antiviral activity, including against Zika virus (half-maximal effective concentration [EC50] = 21.4 μM) [26], hepatitis B virus (half-maximal inhibitory concentration [IC50] = 0.11 μM) [27], Japanese encephalitis virus (IC50 = 7.0 μM) [28], human coronavirus (HCoV) 229E (IC50 = 0.77 μM) [29], human coronavirus OC43 (IC50 = 0.49 μM) [29], Middle East respiratory syndrome (MERS)-CoV (IC50 = 8.4 μM) [30], and SARS-CoV (IC50 = 1.5 μM) [30]. Given its excellent safety and broad-spectrum antiviral activities, EGCG may contribute to immediate clinical solutions for COVID-19 treatment.
Many studies have reported the impressive effects of EGCG against SARS-CoV-2 in vitro (Figure 1b). For example, Hurst et al. [17] demonstrated that EGCG blocks SARS-CoV-2 infection in Vero 76 cells (EC50 = 0.59 µM), while displaying only mild toxicity (selectivity index [SI] = 8.5). The 3C-like protease (3CLpro, also known as Mpro) is highly conserved among coronaviruses, including SARS-CoV-2 [31,32]. Given its essential role in viral replication and transcription, Mpro represents a promising therapeutic target against coronavirus infection [33]. Du et al. [34] showed that EGCG is a potent inhibitor of Mpro, with an IC50 of 0.87 μM. Surface plasmon resonance binding experiments demonstrated that EGCG has a high binding affinity for Mpro, with a dissociation constant (KD) of 6.17 μM. Similarly, Zhu et al. [35] reported that EGCG inhibits Mpro activity, with an IC50 value of 7.51 µM. Furthermore, Ngwe Tun et al. [36] indicated that EGCG is highly effective at inhibiting SARS-CoV-2 replication (IC50 = 6.5 μM) in Vero E6 cells and with minimal toxicity (SI >154). Mechanistically, the authors further demonstrated that EGCG blocks SARS-CoV-2 replication at both the entry and post-entry stages of infection, and also inhibits SARS-CoV-2 Mpro activity.
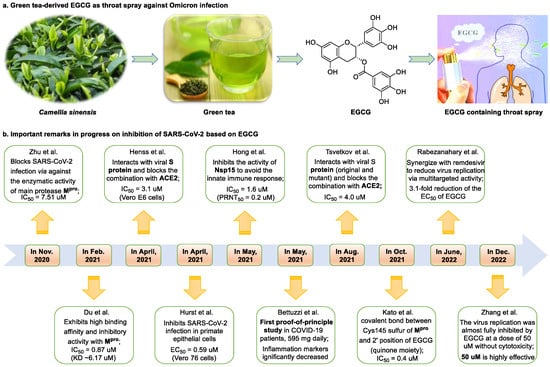
Figure 1. Epigallocatechin 3-gallate is a green tea-derived, multitargeting, anti-SARS-CoV-2 therapeutic candidate. (a) Epigallocatechin 3-gallate (EGCG), isolated from Camellia sinensis, has potential for development as a therapeutic throat spray for Omicron infection. (b) Important discoveries relating to the multi-target effects of EGCG against SARS-CoV-2. Data from references [17,34,35,37,38,39,40,41,42,43].
Meanwhile, Kato et al. [37] showed that EGCG strongly inhibits the activity of Mpro (IC50 = 0.4 μM) via the formation of a covalent bond between Cys145 of the enzyme and the 2′-position of EGCG (Figure 2). Tsvetkov et al. [38] showed that partial EGCG treatment is highly effective at suppressing viral replication (IC50 = 4.0 μM, SI = 6) by interfering with the binding between ACE2 and SARS-CoV-2 spike (S) protein. Similarly, Henss et al. [39] reported that EGCG inhibits SARS-CoV-2 infection (IC50 = 3.1 µM, SI > 11.6) in Vero E6 cells through binding at the SARS-CoV-2 S–ACE2 interface. SARS-CoV-2 endoribonuclease NendoU (NSP15), a uridine-specific endoribonuclease used by the virus to avoid the innate immune response, is considered a compelling drug target [44]. Hong et al. [40] showed that EGCG strongly inhibits the activity of NSP15, with an IC50 value of 1.6 μM. In the same study, the authors investigated the neutralizing effect of EGCG against SARS-CoV-2 and obtained a promising result (half neutralization effect concentration [PRNT50] = 0.2 μM). The above findings regarding the efficacy of EGCG appear to be generalized, indicative of the therapeutic potential of EGCG for the treatment of COVID-19. Meanwhile, combination drug therapy may offer additional advantages [45]. Rabezanahary et al. [41] revealed that the combination of EGCG (15.6 µM) and remdesivir (1.25 µM), the first FDA-approved inhibitor of SARS-CoV-2 RdRp, exerts a significant synergistic effect (3.1-fold reduction in the EC50 of EGCG for RdRp) in Vero E6 cells through multitargeting activity.
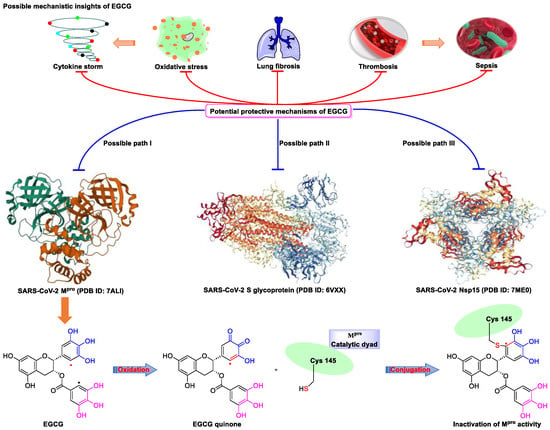
Figure 2. Proposed multi-target (S protein, Nsp15, and Mpro) mechanism of action of EGCG against SARS-CoV-2: inhibition of oxidative stress, cytokine storm, lung fibrosis, thrombosis, and sepsis injury in SARS-CoV-2 infection. Oxidized EGCG is first recognized by the catalytic site of Mpro, which is followed by the covalent bonding between the α,β-unsaturated carbonyl moiety of EGCG (serves as an electrophile) and Cys145 of Mpro (serves as a nucleophile).
Bettuzzi et al. [42] conducted a 15-day, proof-of-principle study to evaluate the anti-SARS-CoV-2 efficacy of EGCG and catechins (two sessions of inhalation plus three capsules daily; total EGCG: 595 mg; total catechins: 840 mg) in 10 non-hospitalized SARS-CoV-2 swab-positive patients. All patients were asymptomatic within 7 to 15 days of starting treatment, while the levels of inflammation markers significantly decreased. No observable adverse events with the EGCG treatment were reported. Additionally, compared with wild-type or Delta strains, Omicron strains have greater replicative capacity in the upper respiratory tract, increasing the likelihood of viral release during breathing; this characteristic may help explain the enhanced transmission of Omicron strains via airborne routes [46]. Yang et al. [47] demonstrated that after drinking two to three cups of green tea, the levels of EGCG in saliva ranged from 4.8 to 22 µg/mL (equivalent to 8.7–39.9 µM), which was two orders of magnitude higher than those in plasma. For cancer prevention, it is recommended that humans consume six cups of green tea daily [47]; accordingly, high doses of EGCG (up to 79.8 μM in saliva) are likely to be safe and may prove highly effective in controlling Omicron infection. Similarly, Furushima et al. [48] investigated the oral retention of catechins in healthy adults after the intake of a beverage (40 mL) containing 73.4 mg of catechins. They found that the average concentrations of EGCG in the oral cavity were approximately 156.3, 58.4, and 50.5 μM at 10, 40, and 60 min, respectively. These findings support the potential value of EGCG as a supplementation therapy for patients infected with an Omicron variant.
SARS-CoV-2 infection can have long-term effects on the lungs as well as on multiple extrapulmonary tissues and organs, while EGCG exerts unique multi-organ protective effects. For example, EGCG plays an important neuroprotective role following traumatic brain injury (through the activation of the adenosine monophosphate-activated protein kinase pathway), [49] ameliorates liver injury secondary to Pseudomonas aeruginosa pneumonia (via upregulating nuclear receptor activation), [50] protects cardiomyocytes against hypoxia–reperfusion injury (via potently inhibiting the self-cleavage of OMA1), [51] and alleviates SARS-CoV-2-triggered cytokine storm, sepsis, thrombosis, and lung fibrosis [52] (Figure 3). In addition, EGCG decreases the severity of Omicron-related COVID-19 symptoms in both elderly patients and patients with metabolic syndrome by downregulating GRP78 expression or promoting hyperinsulinemia remission [43].
Despite its broad-spectrum antiviral activity, favorable safety profile, and multi-organ protective effects, EGCG demonstrated poor oral bioavailability (F) in both rats (F = 0.1%) and humans (F = 0.3%) [53]. Accordingly, the development of an EGCG throat spray as a potential therapeutic strategy targeting Omicron infection should be further explored in the clinical setting.
3. Myricetin—A Waxberry-Derived Covalent Mpro Inhibitor Suitable for Lead Optimization
Myricetin is a well-known nutritional supplement that can be isolated from “medicine food homology” plants, such as Myrica rubra, Ampelopsis grossedentata, Malus domestica, and Cistus monspeliensis [54]. Specifically, vine tea (A. grossedentata), which has myricetin as the main bioactive ingredient, received approval as a functional food ingredient in 2013 and is traditionally consumed worldwide owing to its health-promoting effects and pleasurable taste [55]. Myricetin, a natural dietary flavonol, has numerous pharmacological effects, including improving bleomycin-induced pulmonary fibrosis via the targeting of HSP90β [56], combating methicillin-resistant Staphylococcus aureus-related lethal pneumonia by inhibiting caseinolytic peptidase P [57], ameliorating brain injury and neurological deficits via nuclear factor erythroid 2-related factor 2 activation [58], enhancing immunomodulatory functions [59], and mitigating hepatic fibrosis via the inhibition of the TREM-1-TLR2/4-MyD88 signaling pathway [60]. Myricetin is also an antiviral drug with low toxicity that can treat a wide variety of viral infections in vitro, including Ebola virus (IC50 = 2.7 μM) [61], Marburg virus (IC50 = 25.5 μM) [62], infectious bronchitis virus (IC50 = 10.6 μM) [63], HIV-1 virus (IC50 = 7.6 μM) [64], African swine fever virus (IC50 = 8.4 μM) [65], Bourbon virus (IC50 = 2.2 μM) [66], and herpes simplex virus (IC50 = 1.6 μM) infections [67].
Myricetin is an ideal candidate for research targeting SARS-CoV-2 infection. SARS-CoV-2 helicase (NSP13), a highly conserved non-structural protein possessing RNA helicase and 5′-triphosphatase activities, is a promising target for the development of novel anti-SARS-CoV-2 drugs [68].
This entry is adapted from the peer-reviewed paper 10.3390/nu15153443
This entry is offline, you can click here to edit this entry!
