Integrating bioinspired materials and electrochemical techniques promote specific, rapid, sensitive, and inexpensive biosensing platforms. The selection of biomaterials to decorate a biosensor surface is a critical issue as it strongly affects selectivity and sensitivity. In this context, smart biomaterials with the intrinsic self-assemble capability like bacterial surface (S-) layer proteins are of paramount importance. Indeed, by forming a crystalline two-dimensional protein lattice on many sensors surfaces and interfaces, the S-layer lattice constitutes an immobilization matrix for small biomolecules and lipid membranes and a patterning structure with unsurpassed spatial distribution for sensing elements and bioreceptors. Thus, exploiting S-layer proteins for biosensor technology has already led to various applications ranging from detection of metal ions over small organic compounds to cells. Furthermore, enzymes immobilized on the S-layer proteins allow specific detection of several vital biomolecules. The special features of the S-layer protein lattice as part of the sensor architecture enhances surface functionalization and thus may feature an innovative class of electrochemical biosensors.
- S-layer proteins
- electrochemical biosensor
- biocompatible layer
- self-assembly
- bioinspired material
Biosensors are analytical devices that contain an integrated set of tools for the conversion of a biological response or an analyte concentration to specific (semi)quantitative information [[1]]. A typical biosensor is composed of three main parts (Figure 1): (i) Biorecognition elerments that specifically recognize an analyte and capture it; (ii) an interface matrix, which is a functional area where reactions occur; and (iii) a physico-chemical transducer element that converts the generated event into a (mostly electronic) quantifiable signal [[1],[2]]. The detector unit can amplify the transducer signal before its transmission for processing and analysis by computer software. Several transduction mechanisms depend on the conversion of optical, acoustic, electrical, chemical, mechanical, or thermal properties, or a combination of different mechanisms, to detect biorecognition events [[1],[2]]. All these techniques have led to the development of biosensors that are currently on the market and there is a great deal of interest in developing devices applicable as diagnostic tools for, e.g., point-of-care (POC) testing. The main obstacles for the development of POC sensing devices with high sensitivity and selectivity are the complexity of the transduction principles and high production costs. Moreover, patients cannot use the POC devices by themselves, as skillful technicians are necessary to operate these instruments.
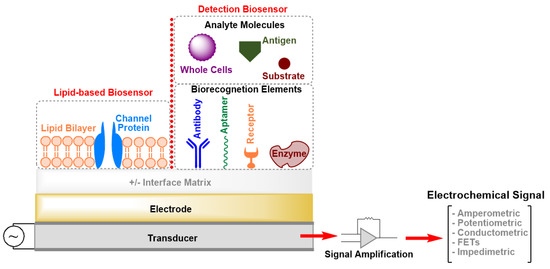
Figure 1. Schematic drawing (not drawn to scale) of the elements of an electrochemical biosensor. FETs: Field-Effect Transistors.
Electrochemical biosensors offer many advantages, including simplicity, low cost, short measurement time, requirement for only small volumes of analyte and reagents, and high selectivity and sensitivity. Moreover, easy operation is feasible that enables patients to handle electrochemical biosensors at home without the need for expensive facilities and personnel. Further, microelectronic circuits can not only be produced at low cost but can also easily connect with common electronic read-out and processing devices [[2],[3][4]]. In contrast, electrochemical biosensors also possess some limitations. For example, although they make it possible to detect a target analyte in a complex biofluid sample, the pH and ionic strength can markedly interfere with the biochemical response signal. Further, the selectivity and sensitivity depend mainly on the architecture of the sensing layers. The design of the latter and the content of the biological recognition elements that are integrated within or connected to a transducer dominate the response to a specific analyte and subsequently control the biochemical event [[2],[5]]. Exploiting (nano)biomaterials for functionalizing the sensor surface can significantly improve its sensitivity, especially to low-molecular-weight analytes and its signal-to-noise ratio. However, these materials must also satisfy particular criteria, such as being stable, flexible, biocompatible, biodegradable and must not interact with the analyte and surrounding biological molecules to avoid any undesired physical–chemical interactions [[6]]. Promising biomolecules are surface (S-) layer proteins, which cover the surface of many prokaryotic cells with a closed lattice [[7][8]]. The most significant characteristics of S-layer proteins for technological application is the intrinsic ability of natural and recombinant protein subunits to self-assemble on surfaces or interfaces into crystalline arrays. These highly porous lattices reveal many biological functions including a remarkable antifouling characteristics [[7]], which can be utilized to avoid passivation of electrochemical biosensors.
This review highlights the general approaches for the development of electrochemical biosensors and focuses on the exploitation of S-layer proteins to functionalize the sensing surface. The S-layer lattice constitutes a biocompatible intermediate layer for linking biological molecules to the electrode to decouple biological molecules from the inorganic surface (Figure 2) [[9][10]]. This is of utmost importance as biological molecules may lose their structure and function on inorganic surfaces. The pores of the S-layer lattice allow unhindered electron transfer from and to the electrode surface and additionally provide an ion reservoir. Finally, the S-layer protein lattice acts as a versatile scaffolding for the formation of supported lipid membranes. The latter provide the essential ambience for the insertion of membrane-active peptides and reconstitution of membrane proteins as highly sensitive and specific sensing elements.
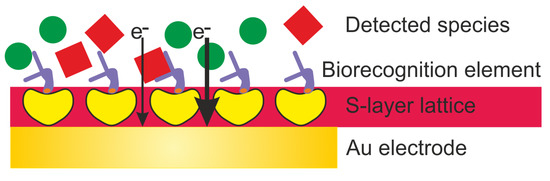
Figure 2. Schematic drawing (not drawn to scale) of an electrochemical biosensor with an S-layer lattice as intermediate layer for linking biorecognition elements to the Au (gold) electrode surface.
This entry is adapted from the peer-reviewed paper 10.3390/s20061721
References
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S.; Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131, .
- Dorothee Grieshaber; Robert MacKenzie; Janos Vörös; Erik Reimhult; Electrochemical Biosensors - Sensor Principles and Architectures. Sensors 2008, 8, 1400-1458, 10.3390/s80314000.
- O. Siggaard-Andersen; Biosensors in Emergency Clinical Chemistry. Scandinavian Journal of Clinical and Laboratory Investigation 1988, 48, 72-72, 10.1080/00365518809168512.
- Elif Burcu Bahadır; Mustafa Kemal Sezgintürk; Electrochemical biosensors for hormone analyses. Biosensors and Bioelectronics 2015, 68, 62-71, 10.1016/j.bios.2014.12.054.
- Xueqing Zhang; Qin Guo; Daxiang Cui; Recent Advances in Nanotechnology Applied to Biosensors. Sensors 2009, 9, 1033-1053, 10.3390/s90201033.
- Eggins, B. Chemical sensors and biosensors. In Analytical Techniques in the Sciences; John Wiley and Sons: Hoboken, NJ, USA, 2002.
- Uwe B. Sleytr; Bernhard Schuster; Eva-Maria Egelseer; Dietmar Pum; S-layers: principles and applications. FEMS Microbiology Reviews 2014, 38, 823-864, 10.1111/1574-6976.12063.
- Dietmar Pum; José Toca-Herrera; Uwe B. Sleytr; S-Layer Protein Self-Assembly. International Journal of Molecular Sciences 2013, 14, 2484-2501, 10.3390/ijms14022484.
- Bernhard Schuster; Uwe B. Sleytr; Biomimetic interfaces based on S-layer proteins, lipid membranes and functional biomolecules.. Journal of The Royal Society Interface 2014, 11, 20140232-20140232, 10.1098/rsif.2014.0232.
- Bernhard Schuster; S-layer Protein-based Biosensors. 2018, , , 10.20944/preprints201803.0071.v1.
