Nanoparticles are defined as matter that has at least one dimension between 1 and 100 nm, and that generally has different properties from its bulk. This is particularly the case for metal nanoparticles, such as gold nanoparticles (NPs), which have a different color depending on their size and differs from the color of bulk gold, in connection with their surface plasmon resonance. Gold NPs are widely used for diagnostics and therapy. Dendrimers are monodisperse hyperbranched polymers of nanometric size, very different from classical polymers, as they are synthesized step-by-step and not by bulk polymerization reactions. Most dendrimers are synthesized by a divergent process, starting from a multifunctional core, possessing 2 to 8 functions in most cases. Such a divergent process frequently involves two steps. Among the different types of dendrimers, polyphosphorhydrazone (PPH) dendrimers have been chosen.
- dendrimers
- nanoparticles
- hybrid materials
1. Gold Nanoparticles
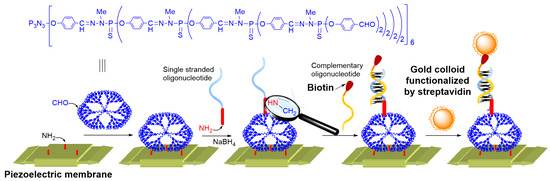
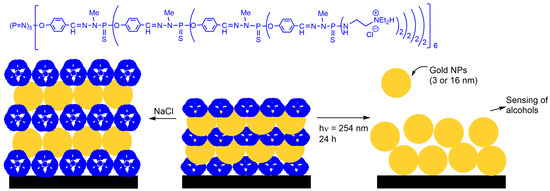
2. ZnCdSe Quantum Dots
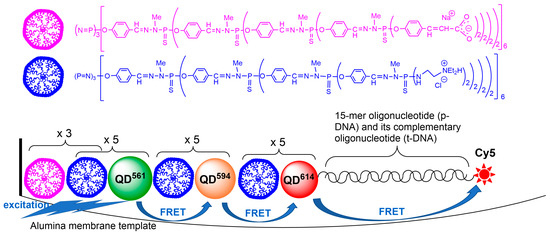
3. Cobalt Nanoparticles
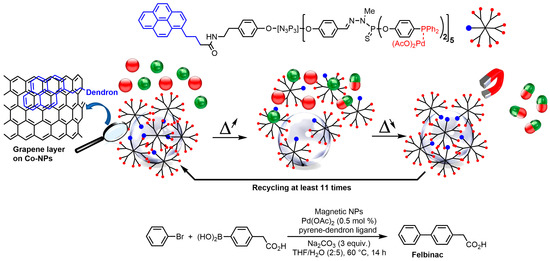
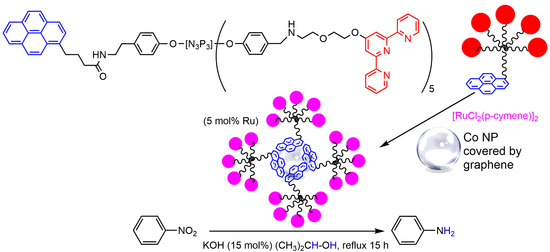
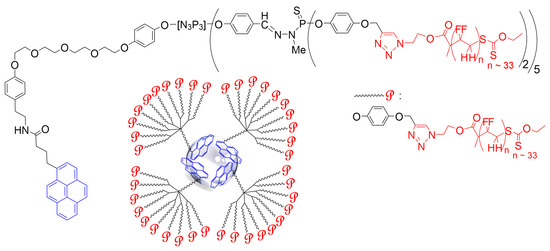
This entry is adapted from the peer-reviewed paper 10.3390/molecules28155739
References
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold nanoparticles: Past, present, and future. Langmuir 2009, 25, 13840–13851.
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Uni.-Sci. 2021, 33, 101560.
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126.
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779.
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294.
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779.
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282.
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782.
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Delivery Rev. 2008, 60, 1307–1315.
- Majoral, J.P.; Zablocka, M.; Caminade, A.-M.; Balczewski, P.; Shi, X.; Mignani, S. Interactions gold/phosphorus dendrimers. Versatile ways to hybrid organic-metallic macromolecules. Coord. Chem. Rev. 2018, 358, 80–91.
- Le Berre, V.; Trevisiol, E.; Dagkessamanskaia, A.; Sokol, S.; Caminade, A.M.; Majoral, J.P.; Meunier, B.; Francois, J. Dendrimeric coating of glass slides for sensitive DNA microarrays analysis. Nucleic Acids Res. 2003, 31, e88.
- Trevisiol, E.; Le Berre-Anton, V.; Leclaire, J.; Pratviel, G.; Caminade, A.M.; Majoral, J.P.; Francois, J.M.; Meunier, B. Dendrislides, dendrichips: A simple chemical functionalization of glass slides with phosphorus dendrimers as an effective means for the preparation of biochips. New J. Chem. 2003, 27, 1713–1719.
- Caminade, A.M.; Padie, C.; Laurent, R.; Maraval, A.; Majoral, J.P. Uses of dendrimers for DNA microarrays. Sensors 2006, 6, 901–914.
- Nicu, L.; Guirardel, M.; Chambosse, F.; Rougerie, P.; Hinh, S.; Trevisiol, E.; Francois, J.M.; Majoral, J.P.; Caminade, A.M.; Cattan, E.; et al. Resonating piezoelectric membranes for microelectromechanically based bioassay: Detection of streptavidin-gold nanoparticles interaction with biotinylated DNA. Sens. Actuator B-Chem. 2005, 110, 125–136.
- Diamandis, E.P.; Christopoulos, T.K. The biotin (stept)avidin system-Principles and applications in biotechnology. Clin. Chem. 1991, 37, 625–636.
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin-biotin technology: Improvements and innovations in chemical and biological applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353.
- Heinisch, T.; Ward, T.R. Artificial metalloenzymes based on the biotin-streptavidin technology: Challenges and opportunities. Acc. Chem. Res. 2016, 49, 1711–1721.
- Kim, D.H.; Karan, P.; Goring, P.; Leclaire, J.; Caminade, A.M.; Majoral, J.P.; Gosele, U.; Steinhart, M.; Knoll, W. Formation of dendrimer nanotubes by layer-by-layer deposition. Small 2005, 1, 99–102.
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743.
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 2001, 13, 1389–1393.
- Zhao, W.B.; Park, J.; Caminade, A.M.; Jeong, S.J.; Jang, Y.H.; Kim, S.O.; Majoral, J.P.; Cho, J.; Kim, D.H. Localized surface plasmon resonance coupling in Au nanoparticles/phosphorus dendrimer multilayer thin films fabricated by layer-by-layer self-assembly method. J. Mater. Chem. 2009, 19, 2006–2012.
- Lebedeva, O.V.; Kim, B.S.; Vasilev, K.; Vinogradova, O.I. Salt softening of polyelectrolyte multilayer microcapsules. J. Colloid Interface Sci. 2005, 284, 455–462.
- Blais, J.C.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. MALDI TOF mass spectrometry for the characterization of phosphorus-containing dendrimers. Scope and limitations. Anal. Chem. 2000, 72, 5097–5105.
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Graded-bandgap quantum-dot-modified nanotubes: A sensitive biosensor for enhanced detection of DNA hybridization. Adv. Mater. 2007, 19, 1933–1936.
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Functional quantum-dot/dendrimer nanotubes for sensitive detection of DNA hybridization. Small 2008, 4, 566–571.
- Knoll, W.; Caminade, A.M.; Char, K.; Duran, H.; Feng, C.L.; Gitsas, A.; Kim, D.H.; Lau, A.; Lazzara, T.D.; Majoral, J.P.; et al. Nanostructuring polymeric materials by templating strategies. Small 2011, 7, 1384–1391.
- Keller, M.; Colliere, V.; Reiser, O.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Pyrene-tagged dendritic catalysts noncovalently grafted onto magnetic Co/C nanoparticles: An efficient and recyclable system for drug synthesis. Angew. Chem. Int. Ed. 2013, 52, 3626–3629.
- Senapati, K.K.; Roy, S.; Borgohain, C.; Phukan, P. Palladium nanoparticle supported on cobalt ferrite: An efficient magnetically separable catalyst for ligand free Suzuki coupling. J. Mol. Cat. A-Chem. 2012, 352, 128–134.
- Keller, M.; Hameau, A.; Spataro, G.; Ladeira, S.; Caminade, A.M.; Majoral, J.P.; Ouali, A. An efficient and recyclable dendritic catalyst able to dramatically decrease palladium leaching in Suzuki couplings. Green Chem. 2012, 14, 2807–2815.
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822.
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483.
- Asri, H.; Dautel, O.; Ouali, A. Terpyridine-Ru complexes noncovalently supported on cobalt magnetic nanoparticles for nitroarene transfer hydrogenation. ACS Appl. Nano Mater. 2020, 3, 11811–11818.
- Kainz, Q.M.; Reiser, O. Polymer- and dendrimer-coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc. Chem. Res. 2014, 47, 667–677.
- Folgado, E.; Guerre, M.; Mimouni, N.; Colliere, V.; Bijani, C.; Ching, K.M.C.; Caminade, A.M.; Ladmiral, V.; Ameduri, B.; Ouali, A. pi-stacking interactions of graphene-coated cobalt magnetic nanoparticles with pyrene-tagged dendritic poly(vinylidene fluoride). ChemPlusChem 2019, 84, 78–84.
