Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Virology
The coronavirus disease 2019 (COVID-19) pandemic is a global pandemic caused by severe acute coronavirus 2 syndrome (SARS-CoV-2). Although “COVID-19 is now an established and ongoing health issue which no longer constitutes a public health emergency of international concern”, according to the WHO International Health Regulations Emergency Committee, it continues to have major health, economic, and social consequences worldwide.
- COVID-19
- SARS-CoV-2 variants
- vaccines
- effectiveness
- efficacy
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a global pandemic caused by severe acute coronavirus 2 syndrome (SARS-CoV-2). Although “COVID-19 is now an established and ongoing health issue which no longer constitutes a public health emergency of international concern”, according to the WHO International Health Regulations Emergency Committee, it continues to have major health, economic, and social consequences worldwide [1,2]. Despite stringent control and measures having been implemented globally, and the application of innovative vaccines, the pandemic persisted for a long time (almost three years) because of the development of numerous SARS-CoV-2 variants with enhanced transmission and immune evasion [3].
At the beginning of the pandemic, there were three types of SARS-CoV-2 variant classification and definition by the Center for Disease Control and Prevention (CDC)—Variants of Interest, Variants of Concern, and Variants of High Consequence [4]. To avoid confusion and geographical stigmas, the World Health Organization (WHO) proposed that the coronavirus variants receive Greek names—Alpha, Beta, Gamma, Delta etc., from the Greek alphabet. These names will not replace the scientific labels but help the public and non-experts to differentiate between them.
Pandemic prevention measures such as the use of masks, physical and/or social distancing, the testing of symptomatic individuals, and contact tracing have proven only somewhat successful in preventing the transmission of the virus. Therefore, rapid vaccine development seemed to be the best possible option to minimize the morbidity and mortality associated with COVID-19. It turned out that the early approval of the vaccines was able to play a critical role in controlling the COVID-19 pandemic.
Although vaccines have saved millions of lives, early approval requires rigorous evaluation of their safety and efficacy [5]. The lack of data on mRNA vaccines for use on humans and their approval without long-term clinical follow-up is justified because the first clinical trials demonstrated their efficacy and satisfactory safety, according to the established and accepted criteria [6].
It is known that developing and administering a vaccine usually takes many years. The approved RNA vaccines against COVID-19 are inexpensive and easily adaptable, and their production process offers flexibility. One of the best benefits of RNA platform technology is the ability to change nucleotide sequences under the same conditions in the same place of manufacture to combat emerging mutants that evade immune system responses. Thus, mRNA vaccines may provide broad protection against different SARS-CoV-2 VOCs (variants of concern) [7].
Aside for vaccination, different therapeutic techniques such as immunotherapy and antiviral medications were and still are among the strategies to prevent infection and successfully limit the virus’s spread. Current forms of vaccinations that are used to prevent and cure COVID-19 include inactivated, viral vector, DNA, and mRNA vaccines [8]. Even last fall, two nasal COVID-19 vaccination formulations were approved for use in India and China, although their approval has not yet been applied for in Europe. These contain modified adenoviruses that are self-attenuating and, according to the results of the Phase 1 clinical trial, they provide strong protection against SARS-CoV-2 infection [9,10]. In addition, mucosal vaccines against COVID-19 continue to be discussed and developed as potential players in controlling the pandemic [11,12,13].
The number of vaccines in pre-clinical development is 199, and in clinical development—183 [14]. Currently, more than 10 vaccines against COVID-19 have been authorized under an emergency use authorization (EUA), such as Pfizer-BioNTech’s BNT162b2, Moderna’s mRNA-1273, AstraZeneca’s ChAdOx1 nCoV-19, Janssen’s Ad26.COV2.S, Sinovac’s vaccine, Sinopharm’s vaccine, CanSino’s Ad5-nCoV vaccine, ZF2001, Sputnik V’s vaccine, the EpiVacCorona vaccine, CureVac, and BBV152 [3,15,16]. Current data on available vaccines against COVID-19 and their efficacy in clinical trials are discussed below.
As of 22 May 2023, over 13 billion vaccine doses have been administered [17]. However, 70% of the world’s population has received at least one dose, in contrast to only 29.9% of low-income countries’ populations [18].
The high worldwide incidence increases the viral burden of SARS-CoV-2 in populations, raising the likelihood of new mutations. Mutations in viruses are obtained at different levels and depend on various factors: the cellular environment, the replication mechanism, polymerase enzyme action, the ability of a virus to correct mismatches by proofreading or post-replicative repair, regulation, etc. [19,20,21,22]. SARS-CoV-2 also mutates for different reasons that are related to its natural environment and external selective pressure. Natural selection plays a vital role in the occurrence of new mutations. Mutations that amplify the reproduction, transmission, and immunity escape response could continue to increase if they help the viruses survive [10].
More than 4000 mutations have been identified in the spike protein of the SARS-CoV-2 genome since the beginning of the COVID-19 pandemic. Studies of its mutation quantities among geographical areas were performed to analyze samples of amino acid sequences (AAS) for envelope (E), membrane (M), nucleocapsid (N), and spike (S) proteins [23,24]. The data showed that 96.40% of E AASs have no mutation, for M AASs—36.76%, for N protein—2.20%, and for C AAS—2.11%. An analysis for the presence of one mutation showed E—3.56%, M—59.64%, N—5.68%, and C—26.86%, respectively. Two mutations were found in E in 0.02%, M—2.80%, N—7.11%, and C AASs—26.15% [25].
The schematic diagram below shows the SARS-CoV-2 virus particle structure and genome organization with an arrangement of various non-structural, structural, and accessory genes: 5′-cap-UTR-replicase-S-E-M-N-3′UTR-poly (A) tail with accessory genes interspersed among the structural genes are illustrated in Figure 1.
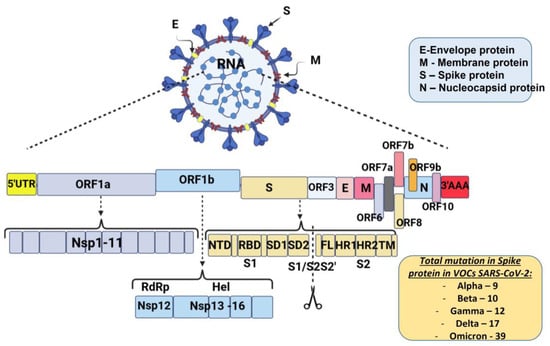
Figure 1. Schematic diagram of severe acute respiratory syndrome coronavirus 2 genome organization: Spike protein (S), membrane protein (M), nucleocaspid protein (N), and envelope protein (E). The genome includes open reading frames (ORFs), 16 non-structural proteins (nsp1–16) encoded by ORF1a and ORF1b, and the accessory proteins among the structural genes. S gene encodes NTD (N-terminal domain), RBD (receptor-binding domain), SD1 (subdomain 1), SD2 (subdomain 2), FL (fusion loop), HR1 (heptad repeat 1), HR2 (heptad repeat 2), and TM (transmembrane domain). Cleavage of the S1/S2 and S2′ site is shown (modified by [26]).
Most mutations that are necessary for virus transmission are in the S-gene. The spike glycoprotein (S) plays a crucial role in SARS-CoV-2 overcoming the species barrier and interspecies transmission from animals to humans; therefore, it is the primary target of selective pressure [25]. The number of mutations in the S protein of SARS-CoV-2 VOCs that occur is also illustrated in Figure 1.
2. Genetic Variants of SARS-CoV-2 Responsible for Severe COVID-19, Higher Mortality and/or Increased Transmission and Morbidity
2.1. Alpha Variant—B.1.1.7—United Kingdom/Kent Variant
In the fall of 2020, the United Kingdom reported a new, genetically different phylogenetic cluster of SARS-CoV-2. These new VOCs of SARS-CoV-2, including B.1.1.7 (Alpha variant according to the WHO), have a vast number of mutations [27] that affect the virus’s function, transmission, and immune system escape [28,29,30,31]. For example, the Alpha variant includes 17 mutations, 14 non-synonymous point mutations, and 3 deletions. Nine are in the Spike (S) protein, which the virus uses to penetrate cells [32]. Among these, N501Y at the RBD enhances the virus’s binding to the angiotensin-converting enzyme-2 (ACE2) receptor [28], P681H increases the transmission [33], and the deletion H69/V70 in the S protein is linked to immune escape [34]. This variant is currently known to have increased mortality, transmissibility, and potentially increased severity based on hospitalizations [28,32,35,36]. However, evidence shows that it does not affect the patient’s susceptibility to EUA monoclonal antibody treatments and minimizes neutralization by convalescent and post-vaccination sera [37,38,39,40,41,42,43].
Regarding infectiousness, the Alpha variant was believed to be 30–50% more contagious than the original SARS-CoV-2 strain. A study published by the CDC showed that Alpha comprised 66% of cases before the Delta variant became predominant [26].
2.2. Beta Variant—B.1.351—South African Variant
The B.1.351 variant (Beta variant) was first detected in Nelson Mandela Bay, Eastern Cape Province of South Africa. It has 21 mutations and can attach more readily to human cells. Nine mutations are in the S protein; some are the same as in the B.1.1.7 variant [44,45]. Despite the similarities between these variants, the evidence suggests that they arose independently. The substitution at position 484 (E484K) in the RBD of the S protein is present in some VOCs and is reported to be “associated with escape from neutralizing antibodies” [46,47,48].
In early 2021, the researchers used B.1.351 in serum from people vaccinated with the Pfizer/BioNTech (BNT162b2) or Moderna (mRNA-1273) vaccines. They found that antibodies in that serum showed reduced neutralizing activity against the mutant, compared with their activity against the original virus [46,49,50]. The transmissibility is established of this variant to be elevated with potentially increased severity and hospitalizations [34,42].
2.3. Gamma Variant—P.1 or B.1.1.28—Japan/Brazilian Variant
In January 2021, two new variants in Brazil were detected, P.1 and P.2. Although they share mutations with the other discovered variants, they seem to have arisen independently. Lineage P.1 (B.1.1.28 or 20J/501Y.V3), the Gamma variant according to the WHO, was first detected in four travelers arriving in Tokyo after visiting Brazil [51]. It has 17 unique amino acid changes, 10 of which are in the S protein [52]. In addition, the N501Y, K417N, and E484K mutations, also found in the Alpha and Beta variants, have been associated with enhanced affinity to human ACE2, and an increased transmissibility and immune escape reaction [27,53]. The P.1 variant caused the widespread infection in Manaus city, but the new lineage was absent in samples from March to November.
The Gamma variant also has a reported potential to cause reinfections, and studies have shown a slight decrease in the efficacy of the currently available vaccines regarding this variant [34]. As mentioned, SARS-CoV-2 variants with the E484K mutation might escape neutralization antibodies from the convalescent plasma and could also increase the reinfection risk [42,53].
According to the WHO, the other variant, P.2, or the Zeta variant, has a notable mutation, E484K [54], but it is no longer detected in different countries or is at very low levels.
2.4. Epsilon Variant—B.1.427/B.1.429—Californian Variant
At the end of 2020, a new variant of SARS-CoV-2 was reported in California [55,56,57]. Both lineages, B.1.427 and B.1.429, carry an identical set of three mutations (W152C; S13I; and L452R) in the ACE2-binding interface of the spike protein [56,57,58]. However, they differ in their additional synonymous and non-synonymous mutations [58]. The University of California reported that variant B.1.427/B.1.429 is four times less susceptible than the original coronavirus to neutralizing antibodies from the blood of people who recovered from COVID-19 and two times less susceptible to antibodies from the blood of people vaccinated with the Moderna (mRNA-1273) or the Pfizer/BioNTech (BNT162b2) vaccines [56,58].
2.5. Eta Variant—B.1.525—Nigerian Variant
Variant B.1.525 was identified in December 2020 in Nigeria and the UK. It has few mutations, which are the same as in the B.1.1.7 Lineage—E484K, deletions ΔH69/V70, and Δ144 in the NTD of the S protein. Together, these increase the transmissibility of SARS-CoV-2 [59].
The critical difference between the B.1.525 variant and other variants is the changes in the S protein, which may be able to attach itself to human cells more effectively in this variant. The B.1.525 has unique S protein mutations such as Q677H, Q52R, A67V, and F888L [59,60].
2.6. Iota Variant—B.1.526—New York Variant
In October 2021, a new variant of SARS-CoV-2, known as B.1.526, was identified in New York City [61,62,63]. The variants carry D614G and A701V mutations in the S protein and several novel point mutations [61,64]. The E484K mutation was also observed in the Iota variant and played a critical role in the loss of the activity of neutralizing antibodies and the convalescent and vaccine sera [41,64]. The other version of B.1.526 has an S477N mutation that may increase its ACE2-receptor binding affinity [62,64].
2.7. Delta Variant—B.1.617.2—India Variant
Lineage B.1.617 was first identified in Maharashtra, India, in October 2020 [65]. Within a few months, the variant was detected in different countries and was named lineage B.1.617. It contains three sublineages—B.1.617.1, B.1.617.2, and B.1.617.3. On May 2021, the sublineage B.1.617.2 (Delta variant according to the WHO) was designated as a VOC because its transmissibility was estimated to be equivalent to that of the Alpha variant [66].
B.1.617.2 has a set of S protein substitutions, and several are also present in other variants of interest/concern. Two critical mutations in the RBD domain, L452R and E484Q, affect the neutralizing antibodies’ evasion [67,68,69]. It is the first strain where these two mutations were seen together.
Preliminary evidence suggested that the B.1.617.2/Delta variant has an increased risk of hospitalization compared to the B.1.1.7/Alpha variant [70]. Additionally, recent studies have shown that the P681R mutation is a specific mutation of this lineage and is responsible for the higher pathogenicity of the B.1.617.2/Delta variant [71].
According to the CDC and some research trials, this variant has a potential reduction in neutralization by some EUA monoclonal antibody treatments and slightly reduced neutralization by post-vaccination [4,38,39,47,69].
2.8. Mu Variant—B.1.621—Nigerian Variant
The WHO identified the Mu variant (B.1.621) as a new SARS-CoV-2 variant of interest on August 30, 2021. For the first time, it was isolated in Columbia in January 2021. The Mu variant harbors eight mutations in the S protein: T95I, YY144-145TSN, R346K, E484K, N501Y, D614G, P681H, and D950N. Several of these mutations are present in other SARS-CoV-2 variants: E484K in Beta and Gamma, P681H and N501Y are shared with Alpha, and D950N is shared with Delta. They can reduce the sensitivity against antibodies induced by natural SARS-CoV-2 infection or vaccination [37,41,42].
The recent research on the sensitivity of the Mu variant to antibodies induced by SARS-CoV-2 infection or vaccination shown that the Mu variant is 12.4-fold more resistant to sera of eight COVID-19 convalescents who were infected during the beginning of the pandemic than the original virus. The Mu variant shows a pronounced resistance to the antibodies elicited by natural SARS-CoV-2 infection and the BNT162b2 mRNA vaccine [72,73].
Further monitoring of the Mu variant was strongly suggested because of its “constellation of mutations that indicate potential properties of immune escape.”
2.9. Omicron Variant (B.1.1.529 Lineage)
In November 2021, a new VOC, B.1.1.529, was identified in South Africa (Botswana). It was designated as the Omicron variant by the WHO [74]. Omicron has more than 30 changes to the S protein, several overlapping with those in the Alpha, Beta, Gamma, or Delta variants [75]. Some other reported mutations are in the envelope, membrane, N-terminal domain of the S protein, nucleocapsid protein, etc. [76,77,78]. Most of these mutations are known to increase transmissibility, viral binding affinity, and antibody escape [55,79,80,81,82].
Initially, Omicron was divided into three lineages (BA.1, BA.2, and BA.3), but later, by mid-2022, two more were identified (BA.4 and BA.5) [72,81,82,83,84,85].
BA.1 and BA.2 share 21 mutations in the S protein. The S proteins of BA.4 and BA.5 are similar to that of BA.2 except for the additional 69–70 deletion [85]. The BA.1 subvariant shares nine common mutations in the S protein with most VOCs, suggesting the possible recombination and origin of the Omicron variant. Among these shared mutations, six common ones were found in the Alpha, three in the Beta, three in the Gamma, and two in the Delta variants [85]. Recent studies compared transmission of the Omicron and the Delta variants and found an increased susceptibility to infection with Omicron compared to Delta, regardless of the vaccination status. A higher transmissibility and immune escape of Omicron were also found compared to the Delta variant [86,87,88,89]. Vaccines are ineffective after two doses, but with triple administration/booster, effectiveness reaches over 70% [90,91].
It is impossible to describe all known variants, so researchers tried to summarize the information about those that contributed to severe COVID-19, higher mortality, and increasing morbidity worldwide. These variants and their important mutations [92,93,94,95,96,97,98] are listed in Table 1.
Table 1. Important mutations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants.
| Variant Classifications | Name (Pango Lineage) | Spike Protein Mutations | Reference |
|---|---|---|---|
| Variants Being Monitored (VBM) | B.1.1.7 (United Kingdom variant) ALPHA | Δ69/70, Δ144Y, (E484K *) (S494P *), N501Y, A570D, D614G, P681H, T716I, S982A, etc. | Rambaut et al. [27], 2020; Liu et al. [29], 2021; Liu et al. [30], 2021; Tian et al. [31], 2021; Davies et al. [32], 2021 |
| B.1.351 (South Africa Variant) BETA | K417N, E484K, D80A, N501Y, D614G, D215G, L18F, 241del, 242del, 243del, A701V etc. | Karim [44], 2020; Callaway [45], 2021; Jangra et al. [48], 2021; Greaney et al. [49], 2021 | |
| P.1 (Japan/Brazilian variant) GAMMA | K417N/T, E484K, L18F, N501Y, D614G, T20N, P26S, D138Y, R190S, H655Y, T1027I etc. | Sabino et al. [53], 2021 Voloch et al. [54], 2021; Pearson et al. [95], 2021; | |
| B.1.427/B.1.429 (Californian variant) EPSILON | L452R, D614G, S13I, W152C etc. | McCallum et al. [56], 2021; Tchesnokova et al. [57], 2021; Peng et al. [58], 2021 | |
| B.1.525 (Nigerian variant) ETA | A67V, Δ69/70, Δ144, E484K, D614G, Q677H, F888L, etc. | Public Health England, 2021 (https://www.gov.uk (accessed on20 June 2023)) [66]; Ozer et al. [60], 2022 | |
| B.1.526 IOTA | Spike: (L5F *), T95I, D253G, (S477N *), E484 *, D614G, (A701V *) etc. ORF: L3201P, T265I, Δ3675, P314L, etc. | Annavajhala et al. [61], 2021; Lasek-Nesselquist et al. [62], 2021; West et al. [63], 2021; Zhou et al. [64], 2021 | |
| B.1.617.1 KAPPA | Spike: (T95I), G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H etc. | https://www.gisaid.org/hcov19-variants (accessed on 20 June 2023) [75]; https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 20 June 2023) [92] | |
| B.1.617.3 | Spike: E484Q, T19R, G142D, L452R, D950N, D614G, P681R, etc. | https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html (accessed on 20 June 2023) [4] | |
| B.1.621 MU | T95I, YY144-145TSN, R346K, E484K, N501Y, D614G, P681H, D950N | https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html (accessed on 20 June 2023) [4]; Collier et al. [37], 2021; Wang et al. [41], 2021; Wang et al. [42], 2021 | |
| P.2 ZETA | Spike: E484K, D614G, V1176F; ORF: L3468V, L3930F, P314L; N: A119S, R203K, G204R, M234I etc. | Uriu et al. [72], 2021; Public Health, England, 2021, https://www.gov.uk (accessed on 20 June 2023) [66]; Pearson et al. [95], 2021 | |
| B.1.617.2 DELTA | T19R, (G142D), 156del, 157del, R158G, L452R, T478K, D614G, P681R, D950N, T478K, W258L, 213-214del, A222V, K417N, etc. | https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 20 June 2023) [94] https://www.gisaid.org/hcov19-variants (accessed on 20 June 2023) [75]; Public Health England, 2021, https://www.gov.uk (accessed on 20 June 2023) [59] | |
| Variants of Concern (VOC) | B.1.1.529 OMICRON | Δ69/70, T95I, V143del, G339D, K417N, T478K, N501Y, H655Y, N679K, L981F, Y505H, S373P, S375F, S477N, N440K, Q493R, T347K, D796Y, E484A and P681H, etc. | https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 20 June 2023) [4]; Aleem et al. [76], 2022; Karim et al. [78], 2021 |
| Other variants | R.1 | E484K, D614G, G769V, W152L; ORF: A2584T, P314L, G1362R, P1936H etc. | Cavanaugh et al. [96], 2021 |
| A.23.1 | F157L, P26S, V367F, P681R, R102I, Q613H; NSP: E95K, M86I, L98F, ORF: L84S, E92K etc. | Bugembe et al. [97], 2021; Gómez et al. [98], 2021; https://www.gisaid.org (accessed on 20 June 2023) [75] | |
| B.1.1.318 | E484K, Δ144, other mutations | Public Health England, 2021, https://www.gov.uk (accessed on 20 June 2023) [66] | |
| B.1.324.1 | E484K, N501Y, other mutations | Public Health England, 2021, https://www.gov.uk (accessed on 20 June 2023) [66] | |
| P.3 | E484K, N501Y, other mutations | Public Health England, 2021, https://www.gov.uk (accessed on 20 June 2023) [66] |
* Detected in some sequences but not all.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines11071181
This entry is offline, you can click here to edit this entry!
