Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Environmental
Bioenergy has emerged to be among the primary choices for the short- and medium-term replacement of fossil fuels and the reduction in greenhouse gas (GHG) emissions. The most practical method for transforming biomass into biofuel is thermochemical conversion, which may be broken down into combustion, torrefaction, pyrolysis, hydrothermal liquefaction, and gasification. In this study, producing biofuels using a biomass pyrolysis process was investigated.
- integrated system
- pyrolysis methods
- parameters
- simulation
1. Pyrolysis Classifications
There are three distinct pyrolysis methods, namely, slow pyrolysis, fast pyrolysis, and flash pyrolysis [31,32]. Table 1 compares and contrasts these processes, highlighting the variations between them in terms of temperature, solid residence time, heating rate, biomass particle size, and product yield. The type of procedure and the process operating parameters determine the product distribution [33]. Figure 1 shows the majority percentages of each product, which are bio-oil, bio-char, and gas.
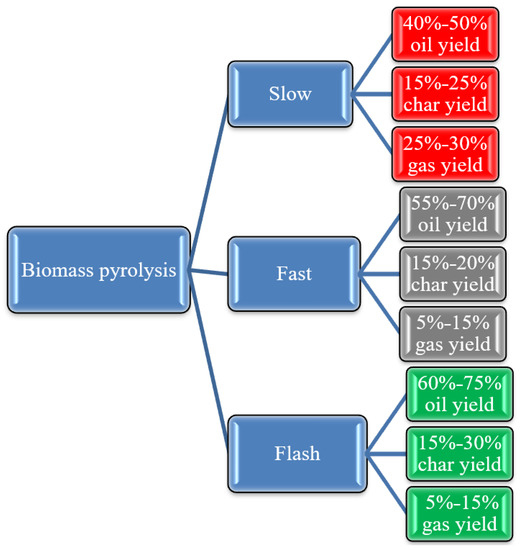
Figure 1. Yield of pyrolysis process at different conditions.
Table 1. Different parameters of the pyrolysis process.
| Parameters | Slow Pyrolysis | Fast Pyrolysis | Flash Pyrolysis | Reference |
|---|---|---|---|---|
| Temperature (°C) | 550–950 | 850–1250 | 900–1200 | [33] |
| Heating rate (°C/s) | 0.1–1.0 | 10–200 | >1000 | |
| Residence time (s) | 300–550 | 0.5–10 | <1 | |
| Particle size (mm) | 5–50 | <1 | <0.5 |
2. Parameters That Affect the Pyrolysis Process
Temperature, feed particle size, residence time, biomass type, catalyst, heating rate, and pressure are all important parameters that affect the pyrolysis process. Both the primary and secondary processes involved in the degradation of biomass during pyrolysis need heat and mass transport. Primary degradation occurs at low temperatures (about 200–300 °C) and involves the breakdown of the complex biomass structure. This process produces intermediate products, such as volatiles, char, and bio-oil. The principal products differ based on the biomass mix and the pyrolysis conditions. Volatiles are gases and vapors that are produced during pyrolysis. Char is the solid residue that is left over after the volatiles have been discharged. It is a carbon-rich, high-surface-area substance that can be utilized as a solid fuel or as a precursor for the synthesis of activated carbon. Bio-oil, commonly known as pyrolysis oil or biomass oil, is a black, viscous liquid produced via volatile condensation. To obtain higher-value products, bio-oil can be improved further using techniques such as hydrotreating or fractional distillation. Secondary degradation occurs at higher temperatures (usually above 500 °C) and is characterized by the thermal cracking of the primary products formed during primary degradation. This process produces more gases, such as light hydrocarbons (e.g., ethylene and propylene), and boosts the overall production of gases and liquids. Secondary decomposition reactions are often faster and more exothermic than primary decomposition events. Syngas, also known as synthesis gas, is a carbon monoxide (CO) and hydrogen (H2) mixture that can be created during the secondary breakdown of biomass. Secondary biomass breakdown can generate several additional gases and vapors, including higher hydrocarbons, tars, and light oxygenates. These products can be developed and treated further for specialized uses. Several parameters influence both the primary and secondary degradation processes in pyrolysis, including the temperature, heating rate, residence duration, and composition of the biomass feedstock. It is feasible to modify the pyrolysis process to produce desired product yields and qualities for a variety of applications, including bioenergy, biofuels, and bio-based compounds, by optimizing these parameters. The breakdown of lignin, cellulose, and hemicellulose into simpler compounds is an example of a primary reaction. The decomposition of intermediates is a key feature of secondary reactions. First, the major products must be broken down into smaller molecules so that cellulose may be converted into sugars; second, the transformation of the main products into huge molecules and char takes place [43].
2.1. Temperature
The pyrolysis process relies on several factors, one of which is temperature. A variety of temperature values are required for the breakdown and devolatilization of biomass constituents. Heavily tarred substances result from the breakdown of hemicellulose and non-condensable gases at temperatures below 300 °C. When biomass is heated to temperatures above 550 °C, it breaks down into its parts and releases several different chemical substances [43]. Acetic acid, levoglucosan, hydroxy-acetaldehyde, and 5-hydromerthylfurfural are all cellulose-derived chemicals, whereas phenolics were derived from lignin [35].
The ideal temperature for producing bio-oils in high yields during fast pyrolysis was reported to be between 400 and 500 °C. The char yield decreased as the pyrolysis temperature increased. Additionally, it was found that additional devolatilization of the primary char balances the generation of secondary char at higher temperatures. At temperatures between 300 and 400 °C, around 80 to 90% of the entire bulk conversion was achieved [48]. The final pyrolysis temperature has a considerable impact on the content and oil of the liquid effluent. Another study showed that a higher temperature resulted in higher biomass conversion efficiencies because more energy was needed to break down the cellulose bonds at higher temperatures. The rate of the solid cake’s decomposition varied with temperature. When the temperature of the soybean cake was raised from 400 °C to 700 °C, an additional 11.82 wt.% of the material was decomposed [49]. Sunflower cake’s yield at 450–700 °C was 10.7 wt.% [50]. The highest liquid yield was achieved during the pyrolysis process between 500 and 550 °C. Secondary reactions, such as the rate of production of gasses increased when the temperature increased to 600 °C. However, the oil yield increased at around 570 °C, whereas the gas yield increased when the temperature values increased from 430 to 730 °C [51]. Stabler species were generated during secondary breaking when the ultimate pyrolysis temperature was raised, where the functional-group-containing compounds were found. Polycyclic aromatic hydrocarbons, such as pyrene, phenanthrene, and others, were formed and accumulated at higher temperatures. Since dehydration and decarboxylation take place at high temperatures, the bio-oil contents increased while the oxygenated concentration decreased [43].
2.2. Size of Feed Particles
Oil production and quality are both affected by the size of the feed particles used in the process. A common trend in biomass pyrolysis is the preference for smaller particle sizes due to the ease and uniformity with which they heat up. For rotating cone pyrolysis, it was recommended that particles be no more than 20 mm in diameter; for fluid bed systems, this number should be no more than 2 mm; and for a circulating fluid bed, it should be less than 6 mm.
Particles of wood with a diameter of 350 µm were fully pyrolyzed, whereas particles with a diameter of 800 µm were converted at a height of 0.9 m [52]. The maximum yield was achieved at 0.45–0.6 mm for hazelnuts and municipal solid waste (MSW) [53]. At a size of 0.6 to 0.85 mm, rapeseed produced the highest yield [54]. In the pyrolysis examination of orange trash, researchers found three distinct particle size ranges (300–180, 180–150 µm, and <150 µm) [55]. For particles less than 150 µm in size, it was found that the thermal behaviors of the beginning and end of the pyrolysis process were varied. Because of variations in surface area, this variation was the result of heat and mass transport processes. However, the liquid output dropped because the larger biomass particles demand more heat and have a low heat transfer coefficient. Limitations in heat transport resulted in higher activation energy for large particles [56]. A high liquid yield was attained from large particles if these particles differ from one another in terms of characteristics like bulk density and oxygen content of the oil. Liquid products were generated when there was less obstruction. There were fewer reactive species and less energy in the liquid because of the increased oxygen level. If the particle size were to be reduced, it would cost more to complete the pyrolysis process since grinding equipment would be required. The technique is more expensive since it uses energy to reduce the size of a particle from its original, larger form. It was noted that different types of biomasses and pyrolizers produced different particle sizes. Optimizing the liquid product yield required careful consideration of both the pyrolizer and biomass sources selection [43].
2.3. Residence Time
One of the most crucial factors in the production of liquid fuels is the residence time. In pyrolysis, a fraction of a minute or less of residence time is optimal for maximizing liquid production. Low residence times are often favored for producing high-quality bio-oil. In pyrolysis, the yield of the liquid products is increased as time goes on due to a secondary reaction. More time in the reactor could be necessary for complete conversion; however, the best potential yield from the liquid is obtained after a short time. The yields of the liquid products increase due to the short residence period at a lower pressure. By varying the residence period from 15 to 40 min with fir sawdust, the effect of residence time was observed [57]. At 30 min, the product liquid yield was the highest (21.22%). It was noticed that bio-oil production increased with the biomass heating rate, even at durations above 40 s. The quantity of oxygen present during pyrolysis was also a factor in the final quality of the oil produced. Two-step pyrolysis, consisting of pyrolysis and oil generation, was used to reduce the oxygen levels.
To produce liquid products with good quality and yield, the residence time must be optimized [43]. When pyrolyzing raw sorghum bagasse at 525 °C, raising the vapor residence time from 0.2 s to 0.9 s lowered the bio-oil yields from 75% to 57% while simultaneously boosting char and gas yields [58]. Similar to this study, the oil yield from pyrolysis of sweet gum hardwood at 700 °C dropped from 22 wt.% to 15 wt.% when the vapor residence time was increased from 0.7 s to 1.7 s [59]. Product distributions as a function of vapor residence time were studied; however, the relationship between the vapor residence time and pyrolysis temperature, and its effect on yields and quality, needs further investigation [60].
2.4. Types of Biomass
Biomass can be classified into two main groups, namely, vegetable-derived and animal-derived, as shown in Figure 2. Lignocellulosic material is made up of three different components: cellulose, hemicellulose, and lignin, all of which can be found in different proportions. Hemicellulose degrades between 470 and 530 °C, cellulose between 510 and 770 °C, and lignin between 550 and 770 °C. Toward the end, ash is produced from the biomass, which contains trace quantities of inorganic substances, like potassium, sodium, phosphorus, calcium, and magnesium. The final product’s elements are extremely sensitive to their constituents. More cellulose and hemicellulose are present at the outset, leading to greater oil output. All three components have distinct temperatures at which they degrade. Cellulose is crystalline and breaks down faster, whereas lignin is complicated and has a greater degree of polymerization [43]. Stronger structural integrity makes decomposing the lignin more challenging, but it results in a bigger char yield [61]. However, the deterioration of this material may be aided by the application of a high heating rate and temperatures, leading to a greater liquid output. Because of the high volatility and reactivity caused by the presence of such a huge quantity of volatile material, bio-oil production is stimulated [62]. In their study of bio-oil production from rice straw and bamboo sawdust, the biomass with a higher volatile material concentration produced a higher yield [63].
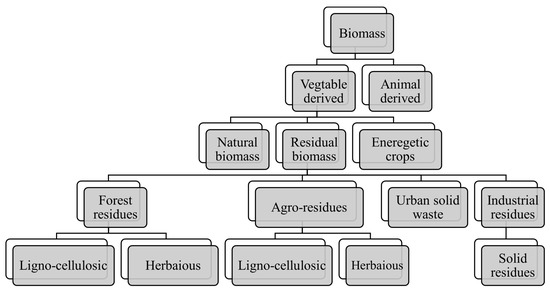
Figure 2. Schematic diagram of biomass types.
The percentages of cellulose, hemicellulose, and lignin in hazelnut shells were 30 wt.%, 23% wt.%, and 38% wt.%, respectively. The percentages of cellulose, hemicellulose, and lignin in farm waste were 17 wt.%, 7 wt.% and 18 wt.%, respectively. Therefore, when lignin levels increased, the bio-oil yields declined but the bio-char yields increased. Agricultural byproducts showed a higher bio-oil output than hazelnut shells.
2.5. Catalyst
Biomass pyrolysis can be conducted with or without catalysts [64]. Catalysts have been utilized to enhance several characteristics of bio-oils, including their ability to be repolymerized, their total acid number, their corrosivity, and their compatibility with petroleum products. Certain pyrolysis processes require specific catalysts [65]. Fluidized and fixed-bed reactors are commonly used in catalytic pyrolysis. These catalysts can be either provided in a solid or vapor phase. The results of the two procedures were distinct because of the differences in contact time and response mechanism. In situ and ex situ pyrolysis improvements of beetle-killed trees were performed in the presence of an HZSM-5 catalyst [66]. More benzene and toluene were produced in the ex situ upgrade, but the specificity for xylenes and aromatics containing carbon 9 was higher in the in situ upgrade. In the presence of a ZrO2-FeOx catalyst, woodchips made from Japanese cedar have a catalytic effect [67]. The ratio of catalyst loading to the feed rate of pyroligneous acid production determined the amount of feed that was converted to ketone. Rapeseed cake was converted gradually between 150 °C and 550 °C, depending on the kind of catalyst utilized. Noncatalytic test (34.06 wt.%) > Na2CO3 (27.10 wt.%) > HZSM-5 (26.43 wt.%) > Al2CO3 (21.64 wt.%) [68] in terms of total organic compounds. Using rapid pyrolysis, desilicated ZSM-5 zeolite was employed as a catalyst for lignocellulosic biomass [69]. The conversion rate and the amount of unwanted coke may both be optimized by carefully regulating the amount of desilicated ZSM-5 utilized in the process. To acquire a large liquid yield, the catalyst choice is crucial. As the catalytic utilization increases, coke generation is reduced, leading to a greater yield of aromatics [43]. By facilitating the dissociation of complex biomass molecules and promoting the production of desired products, catalysts improved the pyrolysis reactions. Catalysts can lower the activation energy required for the pyrolysis reactions, thereby reducing the operating temperature and enhancing the reaction rate. This led to faster and more efficient conversion of biomass into desired products. Catalysts help with increasing the yield of bio-oil, which is a valuable product obtained from biomass pyrolysis. They can promote the cracking of larger molecules, resulting in higher production of lighter hydrocarbons that make up bio-oil. Using certain catalysts can influence the distribution of pyrolysis products, favoring the formation of specific compounds. For example, certain catalysts can promote the production of valuable chemicals, such as furans or phenolic compounds, which have various applications in the chemical industry. Biomass pyrolysis can sometimes lead to the formation of coke, which is a solid carbonaceous residue. Catalysts can suppress coke formation by catalyzing secondary reactions that consume coke precursors or by promoting the gasification of carbonaceous species. Another undesired product that can form during biomass pyrolysis is tar. Tar is a complex mixture of high-molecular-weight compounds that can be problematic in bio-oil production and utilization. Catalysts can help with reducing tar formation during pyrolysis by catalyzing tar-cracking reactions or by promoting secondary reactions that convert tar into more desirable products. Some catalysts can be regenerated and reused, allowing for multiple cycles of biomass pyrolysis. This can contribute to the economic feasibility of the process by reducing catalyst costs and improving the overall process sustainability. Catalysts can enhance the pyrolysis reactions by facilitating the breakdown of complex biomass molecules and promoting desirable product formation. Cracking compounds with greater molecular weights into lighter hydrocarbon products is a common method utilized with catalysts to improve the kinetics of pyrolysis reactions [70]. However, the distributions of products produced by various catalysts vary depending on the parameters in which they were used. Pyrolysis catalysts were divided into three categories according to their intended use. The first class was combined with biomass just before it was introduced to the reactor [71]. The second group was introduced into the reactor, allowing for direct interaction with the vapors, solids, and tars [72]. The third set was transferred to a secondary reactor that followed the pyrolysis reactor.
2.6. Heating Rate
The degradation of biomass into products relies heavily on the heating rate. The rapid breakdown of biomass into its parts in fast pyrolysis calls for a very high heating rate. Also, the highest yields of liquid products are achieved with the shortest residence time and highest heating rate. Thus, fewer undesirable chemicals are generated as a result of the shorter contact duration of the secondary reaction. It was proposed that a heating rate of up to 1000 °C/s might be used. Increases in the production of aliphatic and carbonyl chemicals were observed during rapid pyrolysis of coconut biomass [73]. The optimal temperature for maximizing oil production was observed to be influenced by the heating rate. Different heating rates (50 °C/s, 150 °C/s, and 250 °C/s) were applied to the esparto biomass, and the results showed that at 500 °C, the liquid yield was 45 wt.% for the 50 °C/s and 150 °C min−1 conditions, and 57 wt.% for the 250 °C min−1 condition. The ideal temperature increased from 500 °C to 550 °C at a heating rate of 250 °C/s [43]. The formation of volatiles was increased by increasing the heating rate. If the heating rate is high enough, the temperature will rise to its maximum, but if the heating rate is low enough, the temperature will remain at its minimum [74]. High heating rates are associated with high-quality final products because they reduce the amount of water present, stop secondary reactions from occurring, and create less oxygen [43].
2.7. Pressure
In most cases, atmospheric pressure is used for pyrolysis [43]. Researchers concluded that when completing pyrolysis, the pressure is greater than that of the atmosphere, which results in a greater bio-char yield [33]. Char is formed when the pressure is raised, causing the vapors to remain exposed to the carbon-based substance for a longer duration and for secondary carbon to be produced via decomposition [75]. The amount of carbon in bio-char can be affected by the high pressure within the reactor. Increases in the energy density of bio-char result from high-pressure pyrolysis of biomass, which increases the bio-char’s carbon content [76]. Gases, including nitrogen and argon, as well as water vapor, are employed in the pyrolysis process. Nitrogen gas (N2) is a common inert gas. It was found that the yield of liquid oil was unaffected by the existence of inert gas. By doubling the flow rate from 50 cm3 min−1 to 100 cm3 min−1 [77], the liquid yield was increased by 3 wt.%. Nonetheless, a high liquid product yield may be achieved with a minimal gas flow. The higher the gas flow rate, the more gases are produced, as more volatile substances are evaporated. Also, steam can be utilized as a sweep gas. It was noted that the liquid yield improved when steam was used as the sweep gas [78]. If the oxygen content of the gas is processed, it is reduced by decreasing the gas flow rate, and the bulk density of the gas is increased, and thus, more liquid product is produced.
3. Pretreatment of Biomass
Microwave pretreatment is a promising technology for the conversion of lignocellulosic biomass into sustainable biofuels due to its ability to enhance the efficiency of the conversion process. On rare occasions, microwave pretreatment was investigated. Based on a few studies, here are some recent advances in microwave pretreatment [79]. Improvement of enzymatic hydrolysis: Microwave pretreatment improves the efficiency of enzymatic hydrolysis of lignocellulosic biomass. A study showed that microwave pretreatment of corn stover at 180 °C for 10 min resulted in a 70% increase in glucose yield during enzymatic hydrolysis compared with untreated corn stover. Reduction in energy consumption: Microwave pretreatment can reduce the energy consumption required for the conversion of lignocellulosic biomass into biofuels [80]. A study showed that microwave pretreatment of corn stover at 160 °C for 10 min reduced the energy consumption of the subsequent hydrolysis and fermentation processes by 25%. Enhancement of biofuel production: Microwave pretreatment can also enhance the production of biofuels from lignocellulosic biomass [81]. A study showed that microwave pretreatment of corn straw at 200 °C for 10 min resulted in a 26.7% increase in ethanol yield during fermentation compared with untreated corn straw. Optimization of process parameters: The efficiency of microwave pretreatment can be further improved through the optimization of process parameters, such as temperature, time, and power [82]. Another study showed that optimization of the microwave pretreatment parameters for corn stover, such as a temperature of 180 °C, a time of 10 min, and a power of 800 W, resulted in a 72.3 wt.% increase in glucose yield during enzymatic hydrolysis [83].
4. Pyrolysis Product Properties
The pyrolysis of biomass yields three main byproducts: char, gases, and oil, which when cooled to room temperature, condenses into a dark brown viscous liquid. It is between 350 °C and 500 °C where the most liquid is produced [84]. Due to differences in pyrolysis operations, various reactions take place at varying temperatures. At increased temperatures, molecules in the liquid and residual solid were broken down into tiny ones, enriching the gaseous component [85]. The production of charcoal is obtained at a low temperature with a low heating rate approach. The production of liquid products requires a low temperature, high heating rate, and short gas residence time procedure. The production of fuel gas is achieved at a high temperature with a low heating rate and long gas residence time [31].
This entry is adapted from the peer-reviewed paper 10.3390/su151411238
This entry is offline, you can click here to edit this entry!
