You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Wound healing is an intricate physiological process consisting of a series of molecular and cellular events that facilitate the regeneration of the skin, a protective barrier against the external environment. Since its inception, hydrogels have advanced the field of wound healing, insofar as to promote damaged tissue healing within a hydrated milieu. As well, the integration of therapeutic nanoparticles (NP) and biomolecules into hydrogels for local wound application has been shown to enhance and accelerate healing.
- nanoparticles
- nanotechnology
- wound healing
- antimicrobial agents
- hydrogels
1. Nanotechnology for Wound Healing
Nanotechnology is defined as the manipulation of materials on an atomic or molecular scale [1]. Ever evolving, nanotechnology has revolutionized many industries, especially within the fields of nanoscience, nanoparticles, nanomaterials, and nanomedicine. Specifically, the field of nanomedicine has risen in popularity with myriad applications, including vaccine production, wearable devices, implants, drug delivery, and antibacterial applications [2]. In tissue engineering and regenerative medicine, nanomaterials have shown low toxicity and customizability, making them versatile agents to incorporate into medical practice [2]. For instance, metal nanoparticles such as silver (Ag) [3], gold (Au) [4], copper (Cu) [5], and zinc oxide (ZnO) [6] have demonstrated marked antimicrobial properties. While these intrinsic properties are advantageous for wound healing, these metal nanoparticles can also display anti-infective properties within drug-delivery vehicles.
2. Nanoparticles Used in Wound Healing
Metal nanoparticles have been considered in clinical applications for reasons including small size, high surface-to-volume ratio, shape, stability, low toxicity, and economic reasons, given their affordability [7][8]. Additionally, they can conveniently integrate into wound dressings [7]. One of the primary mechanisms in which antibacterial activity is offered by metal nanoparticles is through their bacteriostatic properties via attachment to DNA or RNA, via electrostatic interactions, halting further replication [9]. MicroRNAs, short, non-coding RNA molecules that have regulatory roles in gene expression, play a large role in wound healing processes, including inflammation, angiogenesis, cell proliferation, and ECM remodeling. In aberrant wound healing, such as infectious states, microRNAs can be targeted by metal nanoparticles through encapsulation, shielding charge groups and allowing for cellular uptake [10]. The modulation of microRNA allows for the enhancement of gene expression factors, promoting the production of factors essential for wound healing. Further, targeted delivery of these therapeutic agents minimizes off-target effects [10].
Another mechanism is through bactericidal properties via the creation of reactive oxygen species [9]. When embedded in hydrogel scaffolds, a substitute is created for damaged ECM which facilitates fibroblast proliferation and matrix formation for enhanced regeneration and repair [11][12]. As such, these nanoparticles can be used in lieu of antibiotics and are thought to accelerate and ameliorate healing while preventing infection [7][8]. A graphical summary of wound healing mechanisms per nanoparticle is depicted in Figure 1.
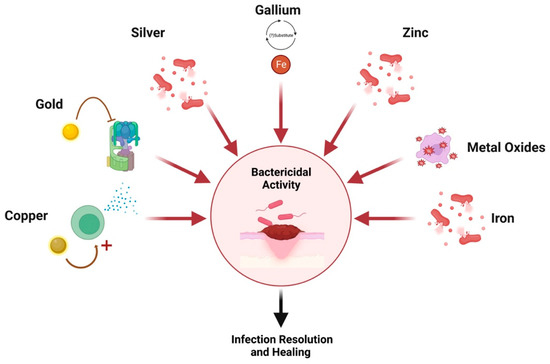
Figure 1. Nanoparticle mechanisms of action. Created with Biorender.com.
2.1. Silver (Ag) Nanoparticles
The use of silver for the treatment of wounds and infection prevention dates back to at least 4000 B.C.E. with documented medical applications dating back to the 1700s; however, in large quantities, silver can also impair healing due to its toxic effects on keratinocytes and fibroblasts [13][14]. Today, silver continues to serve many applications in wound healing. For example, silver nitrate is used as a commonplace treatment for chronic wounds while silver sulfadiazine is used for burns. Nanotechnology has changed the use of silver for wound healing with the creation of silver nanoparticles (AgNPs), which are the most commonly used metal nanoparticles in wound management with many applications, including wound infections, ulcers, and burns. Known for their wide range of antimicrobial activity, effective against bacteria, viruses, fungi, and protozoa, as well as promotion of wound healing, AgNPs have been shown to disturb quorum sensing, effectively reducing biofilm formation [15][16][17].
The antibacterial effect is demonstrated via bactericidal and inhibitory mechanisms. In terms of bactericidal activity, apoptosis is induced in bacteria through AgNP interactions with sulfur and phosphorous-containing proteins, effectively disrupting cell membranes [18]. Moreover, as DNA consists of sulfur and phosphorous, AgNPs act on these bases to destroy DNA, further facilitating the apoptosis of bacterial cells [18]. Moreover, the continuous release of AgNPs, specifically at lower pH whereby acidic environments facilitate the oxidation of AgNPs to Ag+, negatively charged proteins are bound to, allowing for disruption of bacterial cell walls and membrane [18]. Through this mechanism, cell respiration is also disrupted through damage to bacterial mitochondria [19][20]. Despite these cytotoxic effects, which are AgNP-dose- and size-dependent, the proliferation of fibroblasts and keratinocytes is not affected [21]. In terms of inhibitory mechanisms, the presence of AgNPs in the wound environment allows for the formation of reactive oxygen species (ROS), which further disrupt bacterial cell viability through oxidative stress [19][20]. Wound healing is accelerated through these antibacterial properties as microbes can delay all stages of wound healing.
In addition to the antimicrobial properties of AgNPs, they also promote wound healing [22][23][24][25]. Firstly, they assist in the differentiation of fibroblasts into myofibroblasts, which allows for wound contractility [26]. Moreover, they stimulate the proliferation and relocation of keratinocytes to the wound bed [27]. As such, quicker wound epithelialization and scarless wound healing are promoted [26]. Accelerated and complete healing with increased epithelialization was observed in a study wherein an AgNP hydrogel was applied to a partial-thickness cutaneous wound in mice [28][29].
AgNPs also have anti-inflammatory effects through cytokine modulation, reducing levels that allow for decreased lymphocyte infiltration, further enhancing re-epithelialization [5][30]. One study demonstrated a significant reduction in inflammatory cytokines and oxidative stress, effectively promoting healing, while another study in a burn wound model in mice demonstrated reduced interleukin-6 (IL-6) and neutrophils and increased the levels of IL-10, vascular endothelial growth factor, and TGF-ß [3].
The summary of all in vivo studies related to AgNP-loaded hydrogels is shown in Table 1.
Table 1. Summary of Characteristics and Findings of Included Trials for Silver Nanoparticles.
| Hydrogel Species | Particle Size (Diameter) | Dosage of NP | Rate of Release | Antimicrobial Capability | Cell Type | Cytotoxic Effect | Animal Model | In Vivo Findings | Year | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Polyvinyl Alcohol (PVA) | 74.58 nm | 10% w/v | - | Minimum inhibitory concentration 3.13 ug/mL for E. coli and 25 ug/mL for S. aureus | - | - | Full-thickness wounds in rabbits | Wound closure is accelerated with AgNP (12–14 days) compared to control with marketed drug (23 days) | 2019 | [31] |
| PVA and Cellulose | 10–200 nm | 1000 ppm | ~42% after 48 h | More effectively inhibits S. aureus compared to E. coli (92% bacterial reduction) | - | - | Full-thickness wound in mice | Faster healing with minimum scarring was seen in the AgNP group | 2016 | [32] |
| Chitosan | 10–20 nm | 0.1 mg/g | Constant at regular time intervals | Inhibition zone: Pseudomonas aeruginosa: 21.47 ± 0.50 mm; Bacillus cereus:11.52 ± 0.19 mm Staphylococcus aureus: 19.97 ± 0.73 mm; Escherichia coli: 17.30 ± 0.03 mm; Klebsiella pneumonia: 15.50 ± 0.51 mm | Human leukemia cell lines (THP1) | Nontoxic with an IC50 of 151.10 μg/mL at 24 h | P. aeruginosa infected excisional wound model in rats | Faster healing with better scar appearance in AgNP group with lower bacterial counts and enhanced connective tissue production compared to control | 2021 | [33] |
| Guar gum | 8.24 ± 4.20 nm | <0.200 nM | - | Day 12 post-incision colony count (cfu): AgNPs: 20 cfu; commercial antibacterial gel (control): 51 cfu | Human dermal fibroblast cells | Low cytotoxicity with 80% cells viability | Full-thickness wounds in rats | >40% wound healing and 60% antibacterial activity compared to commercial antibacterial gels | 2021 | [34] |
| Poly (ethylene glycol) diacrylate (PEGDA) | - | 5, 25, and 30 mg/mL | 15.2% Ag+ released in 4 h | Bacterial viability of E. coli and S. aureus after 5–10 min of NIR laser exposure is 0 Inhibition zone: 0 mm |
L929 fibroblast cells | >90% cell viability for all groups other than the groups with 50 mg/mL | S. aureus infected excisional wound model in rats | Good sustained anti-bacterial effects observed with greater wound healing response in experimental group 7 days after treatment | 2022 | [35] |
| Polyacrylic acid | - | 0.1% w/v | Effective against S. aureus and E. coli | Murine fibroblast 3T3 cells | Proliferation promoted; low toxicity and good cell viability | Full-thickness wound model in diabetic mice | 97% wound reduction compared to 81% wound reduction for control on day 14 | 2022 | [36] | |
| Chitosan and gelatin | 20–80 nm; mean 36 nm | 1% w/v | - | Highest biofilm eradication noted against S. aureus and P. aeruginosa with AgNP (89 ± 5%) | Mouse embryonic fibroblasts | Low toxicity | Excisional wound splinting model in BALB/c mice | Accelerated and ameliorated wound healing compared to control with enhanced angiogenesis, collagen synthesis, and sebaceous gland/hair follicle regeneration | 2022 | [37] |
| PF127 polymer | 33 nm | 0.1, 0.3, and 1.0 mg | - | Zone of Inhibition: Bacillus cereus (17.7 mm), Escherichia coli (18.7 mm), Pseudomonas aeruginosa (10.3 mm), and Staphylococcus aureus (17.7 mm) Minimum inhibitory concentration and minimum bactericidal concentration: 390–780 µg/mL |
Drosophila melanogaster eggs | No significant effect on eclosion of F1 flies for doses under 250 micrograms/mL of AI-AgNPs | Full-thickness excisional wound model in mice | In a concentration-dependent manner, the wound contraction for the treatment groups were higher than for the control group; no skin irritation observed in patch test | 2021 | [38] |
| Chitosan | 20–35 nm | - | Continuous with release co-efficient of 0.37 | Zone of Inhibition: E. coli: 13.6 ± 0.3 mm; B. subtilis: 10.5 ± 0.8 mm; P. aeruginosa: 9.2 ± 0.3 mm; S. aureus: 11.4 ± 0.1 mm | - | - | Full-thickness excisional wound model in diabetic rats | The rate of wound contraction was higher compared to the control. Scar-free regeneration of the skin with intact patches of hair growth was also noted with the AgNP treatment | 2021 | [39] |
| Polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), carboxymethyl cellulose (CMC) | 31 nm | - | - | - | NCTC L929 cell line | Non-cytotoxic | Full-thickness excisional wounds in rabbits | Stimulatory action on wound healing as evidenced by a high intensity of fibroblasts and neovascularization in the tissue, which promoted a faster healing process when compared to the untreated wounds | 2018 | [40] |
| Gelatin | 68.5 nm | 200 µg/mL | With irradiation: 39.24%; without: 29.92% after 30 min | 22.46% of methicillin-resistant Staphylococcus aureus (MRSA) and 24.48% of E. coli were eliminated compared to 22.46% and 20.37% in the control without irradiation. With irradiation, 97.57% of MRSA and 95.99% E. coli were killed due to photothermal effects of AgNPs | HaCAT cells | At <250 µg/mL, cell viability >80%, however cell viability decreased with increased concentration | MRSA-infected wound model in mice | 91.76% of MRSA in wounds was removed with improved healing, angiogenesis, and collagen deposition. | 2021 | [41] |
| Lignin and cellulose | 100 nm | 3, 6, 9, 12% w/v | - | When in contact with the 12% w/v AgNP hydrogel for 2 h, E. coli, S. aureus, C. albicans, and MRSA had no colonies on the agar plates, indicating that all bacteria that were in contact with the hydrogels were killed | Mouse L929 fibroblast cells | Survival rate of cells in each concentration group was >90% | Full-thickness excisional wound infected with MRSA in rats | The hydrogels can maintain a moist healing environment, reduce inflammatory cell infiltration, promote M2 macrophage polarization, accelerate collagen deposition, promote angiogenesis, and accelerate wound healing of MRSA-infected wounds. | 2021 | [42] |
| Alginate and Gelatin | 7.5–8.3 nm at 1 mM, 20–34 nm at 4 mM | 1.0, 2.0, 40.0 nM | - | Minimum Inhibitory Concentration: 0.50 µg/mL against Pseudomonas aeruginosa and 53.0 µg/mL against Staphylococcus aureus at 4 nm | Human L929 fibroblasts | 96% cell viability observed at 4 mM | Full-thickness excisional wound model in rats | Accelerated wound healing, earlier development, and maturation of granulation tissue | 2020 | [43] |
| Hydroxypropyl methylcellulose | 40–70 nm | 10, 20, 40, 80 µg/mL | - | Inhibition zone increased with increasing concentration. At 80 µg/mL: E. coli: 20 mm; S. aureus: 19 mm | - | - | Full-thickness excisional wounds in rats | The percentage wound healing for formulation was 9.34% more than that of standard (silver sulfadiazine cream) at day 14 with accelerated wound contraction and reduced epithelialization periods, however, the standard showed 1.78% higher healing on day 21. | 2014 | [44] |
| Zwitterionic poly (sulfobetaine methacrylate) monomer and protected dopamine methacrylamide monomers (DMA) hydrogel | 18 ± 2 nm | 2 mM | 46 μg/L for a 5 × 5 cm2 sample during the first day and gradually increased thereafter | Inhibition Zone: 157, 148 and 129% for E. coli, S. aureus, and P. auregenosa compared to control. Less significant effect on Gram-positive than -negative bacteria. Suspension assays measuring the optical density (OD) of bacteria at 600 nm show significantly decreased OD values in AgNP group in comparison to other samples, demonstrating bactericidal effects |
MC3T3-E1, Human HS68/F3T3 Fibroblasts | Due to the low concentrations of silver released, Mammalian cell viability was not greatly affected during 5 days of incubation | Full-thickness excisional wounds in rats | The percentage of the wound size reduction was 59% for the control, 80 for the hydrogel alone, and 98% for the AgNP-hydrogel treatment | 2016 | [45] |
| Pluronic F127 | 2.6 nm | 200 µg/g | Initial burst during the first 10 h, followed by a sustained release over 24 h | AgNPs reduced S. aureus viability by up to 77% compared to 50% in SSD and 20% in the blank hydrogel Zone of Inhibition: AgNP hydrogel: 14 mm; SSD: 11 mm; blank hydrogen: 7 mm |
- | - | S. aureus infected full-thickness excisional wound model in | AgNP hydrogel to the wound provides superior bactericidal activity and reduces inflammation leading to accelerated wound closure when compared to industry-standard silver sulfadiazine. It also accelerated wound closure and improved wound re-epithelialization. Further, decreased neutrophil infiltration, increased anti-inflammatory Ym-1 positive M2 macrophages, and reduced the number of caspase-1 positive apoptotic cells were also observed. | 2021 | [46] |
| Chitosan and Konjac Glucomannan | 60 nm | 200 µg/mL | Release in a gradual manner | Superior ability for AgNPs-loaded hydrogels to kill S. aureus and E. coli. | L929 cells | 95% cell survival rate | S. aureus infected full-thickness wounds in rats | Promotes accelerated infected wound repair with no adverse reactions or symptoms | 2020 | [47] |
| Riclin | 25.92 nm | 1.0 mM | Sustained released with complete release observed at 48 h | Inhibitory concentration of S. aureus was 10 μg/mL and E. coli was 10 μg/mL | Mouse skin fibroblast (NIH3T3) cells and macrophage (Raw 264.7) cells | Weak cytotoxicity (>60% cell viability) toward NIH3T3 mouse fibroblasts at concentrations of 54 μg/mL | S. aureus infected full-thickness wounds in mice | Faster wound healing, more complete re-epithelialization, and denser collagen deposition characteristics. | 2022 | [48] |
| Chitosan | - | 6, 12, 24, 48 mM (24 being the optimum dose) |
After 12 h co-culture, concentrations of 3.01, 3.92, 6.62, and 8.26 mg/L were obtained with Ag+ doses of 6, 12, 24, and 48 mM, respectively |
Significant bactericidal effects noted MPI: S. aureus: 35% and S. epidermis: 34% |
NIH/3T3 cells and KERTr cells | Cell viability could be maintained >90% when the concentration of Ag+ in the HTM was <6 mg/L | Full-thickness wounds in diabetic rats | Higher wound closure efficiency and faster recovery of integrity and functionality of the newly formed tissues compared to other treatments | 2021 | [49] |
| Gelatin | 300 nm | 1 mg/mL | Quick release during the first 48 h, reaching 29.65% In the following stage, prolonged-release profiles over 504 h (21 days) were observed, with a cumulative percentage of 61.37% | A sustained antibacterial effect was observed against E. coli and S. aureus compared to no bacteriostatic ability in the pure hydrogel alone. | MC3T3-E1 | Good cell compatibility observed | Scalded skin model to produce 2-degree burns in rats | Only 15% of the wound area left on day 10. Histology results showed the epidermal and dermal layers were better organized compared to the control. | 2022 | [50] |
| Lignocellulose | - | 0.5 and 0.8% w/v | pH-dependent release | 4.1% survival rate for S. aureus and 2.9% survival rate for E. coli | L929 fibroblast cell line | >95% of the cells are viable after 36 h incubation. No hemolytic activity observed. | S. aureus infected full-thickness wounds in mice | Significantly accelerate tissue regeneration and wound healing process through increasing collagen deposition and decreasing inflammation while retaining excellent biocompatibility | 2021 | [51] |
| Cellulose and gelatin | - | 0.2 and 0.5 mg/mL | - | Decrease in S. aureus and P. aeruginosa activity Inhibition zone: ~2 mm at 0.5 mg/mL |
Neonatal human dermal fibroblasts (NHDF) | CNF/G/Ag0.5 presented highest satisfactory infected cell viability (>100%) | Full-thickness wounds in mice | The CNF/G/Ag groups had much declined size of the wound than the control; wounds treated with CNF/G/Ag0.5 healed ∼90% after treated Bacterial infection of the wound was reflected by weight loss. Treatment with CNF/G/Ag0.5 displayed a clear advantage in survival rate (83.3%) | 2018 | [52] |
| Aloe vera-silk fibroin composite | 40 nm | 0.5 mg/mL | pH-dependent release: 40.89% release in neutral environment, and 55.12% in acidic environment | Antibacterial rings presented in the AgNP hydrogel had the largest diameter both for E. coli (13.92 ± 0.94 mm) and S. aureus (10.623 ± 0.61 mm), demonstrating superior antibacterial properties | L929, Mesenchymal Stem Cells | Promotion of cell proliferation and migration; good biocompatibility | Full-thickness excisional wounds in rats | Accelerating healing and inhibition of immune reactions observed with better performance in early inflammatory response stages. Good antibacterial properties, satisfactory biocompatibility and promotes cell proliferation, migration, and wound healing in the AgNP hydrogel compared to the controls. | 2021 | [53] |
| Cellulose | 119.7 ± 5 nm (natural cashew gum—NCG); 123.8 ± 8.9 nm (phthalated cashew gum—PhCG) | NCG: 36 × 1010 particles/mL PhCG: 4.03 × 1010 particles/mL |
- | Antibacterial activity was tested against S. aureus and P. aeruginosa. The hydrogel base alone did not present an antimicrobial effect. The effect of the hydrogels was more effective against P. aeruginosa, whereas the PhCG-AgNP was more potent than the NCG-AgNPs. For the gram-negative bacterium, the MIC values presented the same value of MBC for both hydrogels, which indicates a bactericidal effect. Hydrogels with AgNPs showed lower MICs when compared to the effect of AgNO3 solutions at the same concentrations tested for the two bacteria. |
- | - | Full-thickness wounds in rats | Improved healing was observed compared to the control | 2017 | [54] |
| Cellulose | 28 nm | - | 2.0% w/v | Good resistance against Gram-positive and Gram-negative bacteria, with E. coli and S. aureus showing superior colony formation suppression. Bacterial death was recorded in 78.9 ± 2.61% of the cases in the control and 95.6 ± 1.93% in the presence of AgNPs |
- | - | S. aureus infected full-thickness wounds in rats | Accelerated wound healing with superior antibacterial and wound healing properties noted. Significantly improved wound closure by day 16, and histological examination of the tissue in the wounded area showed rapid reepithelialisation, differentiated dermis, and epidermis, with minimal scar tissues. | 2022 | [55] |
| Silk fibroin | - | 5% w/v | Rate of release of AgNP varies depending on metformin-loaded mesoporous silica microspheres (MET@MSNs): Ag NPs mass ratios | Colony counts reduced from 7.72 ± 0.10 (CFU/mL) to 6.90 ± 0.09 (CFU/mL) for S. aureus and from 7.15 ± 0.09 (CFU/mL) to 6.30 ± 0.43 for E. coli. Zones of inhibition for S. aureus and E. coli are comparable to antibiotic-sensitive tablets |
RAW264.7, EA.hy926, and L929 cells | For RAW264.7 cells, >90% cell viability on day 7 with hydrogel application | Full-thickness excisional wounds in diabetic mice | Rapid wound healing was observed regeneration of squamous epithelium, collagen formation, and angiogenesis indicative of good wound repair compared to control |
2022 | [56] |
| Carbopol | 21 nm | 100 µg/g | - | Microbicidal activity on S. aureus and E. coli with MBC close to 100 µg/ mL, and MtE has a microbicidal response on S. aureus with MBC of 50 µg/mL. Besides, AgMt NPs-G produces a marked bacterial inhibition by contact in both strains | HUVEC cells | MtE and AgMt NPs tested concentrations for toxicity do not show an important effect on cell viability, except for AgMt NPs 100 µg/mL concentration, where cell viability falls by almost 10% | Second-degree burn injuries in rats | Higher wound healing ratio and faster wound evolution compared to control. |
2021 | [57] |
| Chitosan and polyethylene glycol | 99.1 ± 2.3 nm | - | 0.0055 µg/mL/h | AgNP-impregnated chitosan hydrogels have better antimicrobial potential compared to bare chitosan hydrogel and AgNPs. The zone of inhibition recorded for AgNP-loaded hydrogel against E. coli, P. aeruginosa, B. subtilis and S. aureus were 20.2 ± 1.0, 21.8 ± 1.5, 15.5 ± 0.8 and 21.5 ± 0.5 mm, respectively, which were higher compared to the controls |
- | - | Full-thickness excisional wounds in diabetic-induced rats | The combination of AgNPs and chitosan hydrogel significantly enhances healing. Accelerated wound contraction as well as improved antimicrobial and antioxidant properties were observed compared to the controls. | 2019 | [58] |
| Carbopol | 20 nm | 0.18 mg/g | After 24 h, 10.56 µg/cm2 of AgNPs were released into the skin ex-vivo | - | Murine macrophage (J774A.1), human skin fibroblasts (TE 353.Sk) and human keratinocytes (HaCaT) cell lines | Good cellular uptake; no toxicity 48 h upon exposure | Full-thickness excisional and incisional wounds in rats | Accelerated and enhanced wound healing via modulation of cytokines and growth factors |
2017 | [59] |
| Chitosan and dextran | - | 47 µg/g | The concentration of silver ion released was the highest at the 2 h time point and then declined thereafter | Almost all bacteria (S. aureus and P. aeruginosa) were dead after being treated with the hydrogel/NP after 60 min | NIH 3T3 cells | >80% viability | S. aureus and P. aeruginosa infected full-thickness excisional wounds in diabetic rats | Rapid wound contraction was observed after treatment with the hydrogel, suggesting superior healing activity to promote fibroblast migration, granulation tissue formation, and promotion of angiogenesis. | 2019 | [60] |
| Hyaluronic acid and gelatin | - | 100, 200, 300 µg/mL | - | The bactericidal rates of both E. coli and S. aureus reached > 90% when the AgNPs concentration was 200 μg/mL. The area of the inhibition zone becomes larger with an increase in the AgNPs concentration. | L929 fibroblasts | Good cytocompatibility (cell viability >80% at 24 and 48hr) | Full-thickness excisional wounds in rats and abdominal wall model | The AgNP hydrogel accelerated the healing process by improving wet adhesion, reducing wound inflammation, promoting angiogenesis and formation of granulation tissues compared to the control. | 2022 | [61] |
| Chitosan, ulvan dialdehyde, human umbilical cord mesenchymal stem cell powder | - | 5, 10, 20, 30, 40, 50 mg/mL | - | Zone of inhibition for pure hydrogel was 14 mm and 20 mm for S. aureus and E. coli respectively, compared to 23 mm and 31 mm for AgNP hydrogel | NIH-3T3 fibroblasts | Cell viability >80% with all concentrations | Full-thickness excisional wounds in Type II diabetic mice | Accelerated wound healing observed compared to control | 2022 | [62] |
| Polyvinyl alcohol and chitosan | - | 5 mM | Optimal release at pH 5.7 compared to 7.4. Fastest release in first 3 days, tapering off to ~0.6 ug/mL by day 10. | The AgNP hydrogel demonstrated good bacteriostatic effect after 24 h incubation for both E. coli and S. aureus compared to the pure hydrogel which had almost no bacteriostatic effect. | L929 | No changes in cell viability with L929 cells treated with AgNP hydrogel after day 1, decreased to ~90% by day 2, and ~80% by day 3. | S. aureus infected full-thickness excisional wounds in mice | Accelerated wound contraction and granulation thickness after 10 days compared to control. TNFalpha expression was lowest and VEGF expression was highest in hydrogels containing AgNP in irradiated mice treated with H2O2 in conjunction. | 2022 | [63] |
| Polyvinyl alcohol and alginate | 30–40 nm | 3.18 µg/mL | - | Hydrogels containing Ag-NPs effectively inhibited E. coli and P. aeruginosa from growing. A stronger response was seen with P. aeruginosa than E. coli. | RAW 264.7, human keratinocyte and human dermal fibroblast cell lines | Marginal cell toxicity due to low concentration of AgNP used | Full-thickness excisional wound model in rats | Accelerated wound closure with ameliorated inflammation, enhanced angiogenesis, increased collagen production, and promotion of re-epithelialization compared to control | 2019 | [64] |
| Polyacrylic acid sodium, polyvinyl butyral and chitosan | - | 100, 200, 300 mg/L | The two-stage dressing released < 20% during first 30 min followed by faster release in greater quantities in subsequent period | - | - | As the nano-Ag concentration increased to over 300 mg/L, cell viability was reduced. | S. aureus infected full-thickness excisional wounds in diabetic mice | Accelerated wound healing and reduction of bacteria as well as promotion of re-epithelialization compared to control | 2017 | [65] |
| Polyvinyl alcohol and cellulose | - | 0, 1, 2, 4 mM | - | The bactericidal rates of the AgNP hydrogel at a lower concentration against E. coli and S. aureus were 65.63 ± 2.63% and 51.17 ± 1.49%, respectively. At a higher concentration, the rates were 99.72 ± 0.14% and 99.38 ± 0.48% against E. coli and S. aureus, respectively | L929 cells | The cell survival rate slowly increased with prolonged culture time and ranged from 96% to 134% | Full-thickness excisional wound model in mice | The AgNP hydrogel promoted the growth and development of new blood vessels and significantly accelerated wound healing with their combined antibacterial and anti-inflammatory activities | 2021 | [66] |
| Hyaluronan-polyacrylamide | - | 50, 100, 200 µg/mL | At the first stage, the release of Ag+ showed an exponential increase for the first ten days, followed by a ‘stationary’ phase that continued until the 30th day. | The highest antibacterial effect was exhibited at 200 µg/mL < 1% of the bacteria (E. coli and S. aureus) survived, compared with the control group. No inhibition zone is found in the 0 µg/mL group, whereas clear inhibition zones were observed for the 50, 100, and 200 µg/mL groups towards E. coli and S. aureus | 3T3 cells | Comparable cell viability compared to control after being cultured for 24 h | S. aureus infected full-thickness excisional wound model in rats | The AgNP hydrogel significantly promoted wound healing by improving granulation tissue-formation, angiogenesis, and collagen deposition as well as alleviating inflammation. | 2020 | [67] |
| Hyaluronic acid | 100 nm | 0.5 µM | 90% release within 48 h when the pH was reduced from 7.4 to 5.0 | Robust antibacterial ratios for both S. aureus (95.69%) and P. aeruginosa (86.76%) compared to control | HUVECs and L929 | >75% cell viability maintained over 3 days | S. aureus infected full-thickness excisional wound model in diabetic rats | Accelerated wound closure as well as increased anti-inflammatory, pro-angiogenic, and antibacterial activities were seen in the hydrogel compared to the control. | 2022 | [68] |
| Polyvinyl alcohol and gelatin | - | 0.1, 0.2, 0.3, 0.4 mM | - | Against E. coli and S. aureus, a larger inhibition zone compared to the pure hydrogel. | HaCat, LO2 and 293T cells | No inhibitory effects seen, and at low concentrations, a tendency for cell proliferation was noted (5, 10, 15 µg/mL). At higher concentrations, low inhibition was seen, <20% (20, 30, 50, 100 µg/mL) | S. aureus infected full-thickness excisional wounds in mice | Accelerated wound healing, anti-bacterial properties, reduced inflammation, and increased collagen content compared to control | 2021 | [69] |
| Propyl methacrylate | - | 140, 280, 420 µg/mL | The release amount of Ag+ in Ag2S QDs group was much higher than that in NP hydrogel treatment group. Compared with the NP hydrogel-NIR (−) group, the NP hydrogel-NIR (+) group showed a larger release amount of Ag+ (39.9–84.9 ppb versus 36.6–73.9 ppb), indicating that NIR could accelerate the release of Ag+. NIR = near-infrared radiation |
The inhibition zone diameter of the NP hydrogel group (NP hydrogel-NIR (+) and NP hydrogel-NIR(−)) was significantly larger than that of the control group, with a positive correlation with the NPs concentration against E. coli and MRSA. Additionally, the inhibition zone diameter was obviously larger in the NP hydrogel-NIR (+) group than the NP hydrogel-NIR (−) group, demonstrating that the antibacterial ability of the NP hydrogel was enhanced under the assistance of NIR laser irradiation. | NIH 3T3 MEFs, Vero cells | At 420 µg/mL NP concentration, cell survival was still as high as 93.8 ± 3.7% for Vero cells and 96.8 ± 6.2% for NIH 3T3 cells after 48 h of incubation, demonstrating good cytocompatibility | MRSA infected full-thickness excisional wounds in mice | 9 days of treatment with NP hydrogel could heal the full-thickness skin defects infected with MRSA with enhanced bacterial clearance, significant collagen deposition, upregulation of VEGF expression, and angiogenesis at the infected sites. | 2022 | [70] |
| Copolymer (PEP) | - | 0.75% w/v | - | Good antimicrobial activity against MRSA and E. coli with inhibition zone of 1.3 cm and 1.4 cm, respectively | HUVECs, NIH 3T3 MEFs | for HUVEC, cell viability >98% for 200 µg/mL; for NIH-3T3 cells, cell proliferation was not inhibited over a 3-day span for varying concentrations (50–200 µg/mL) | MRSA infected full-thickness excisional wounds in rats | Rapid wound healing—99.85% of the aggregate wound area was healed over the span of 12 days whereas only 54% wound closure was observed for the untreated group. | 2019 | [71] |
| Chitosan | 60–150 nm | 0.5–6.0 mM | - | More obvious zone of inhibition for S. aureus compared to E. coli. The pure hydrogel alone demonstrated no antibacterial activity | L929 cells | Good cell viability (>90%) for all hydrogels tested | S. aureus infected full-thickness excisional wounds in rats | Accelerated the healing process through anti-infection, anti-inflammation, stimulating collagen deposition, and promoting the formation of epithelia and blood vessels compared to control | 2022 | [72] |
| Methacrylate gelatin | 120 ± 3.392 nm | 20 µg/mL | Day 7, the Ag+ release ratio of the hydrogel was 64.2% ± 4.3% and 70.7% ± 7.8% in solution containing either lysozyme or not, respectively | The numbers of bacterial colony-forming units (CFU) were reduced to 75.3% ± 0.8% for E. coli, 88.8% ± 1.3% for S. aureus, and 82.1% ± 1.4% for P. aeruginosa | NIH 3T3 | Excellent biocompatibility observed | E. coli and S. aureus full-thickness skin burn model in rats | Promotes wound healing by facilitating the regeneration of the epithelial wounds, protecting the wound-rebuilding microvessel network, reducing the inflammation-induced infiltration, enhancing the collagen deposition, and inducing the macrophages to the anti-inflammatory phenotype with noncanonical Wnt signal pathway activated | 2022 | [73] |
| Gelatin and polyvinyl alcohol | 7.4 ± 1.2 nm | - | Sustained release of silver from the hydrogel was detected, and an accumulative 8.99 0.58% of total silver was released after 24 h and 14.98 0.71% was released after 72 h. | >99% of inhibition in all three bacterial strains (99.91 ± 0.52% for E. coli, 99.89 ± 0.35% for S. aureus, and 99.57 ± 0.73% for MRSA) | L929 cells | Similar biocompatibility compared to pure/blank hydrogel | Full-thickness wounds in rats | Improved antibacterial efficacy, accelerated wound healing and rapid re-epithelialization compared to control | 2022 | [74] |
2.2. Gold (Au) Nanoparticles
AuNPs are commonly used in tissue regeneration, wound healing, and drug delivery of bioactive compounds due to their biocompatibility, high surface reactivity, and antioxidative effects [75][76]. While some antimicrobial effects are seen, unlike AgNPs, AuNPs do not offer much antimicrobial activity alone [77].
Antimicrobial action is demonstrated via two principal mechanisms, similar to AgNPs: bactericidal and inhibitory. Cell death is induced via the disruption of ATP synthase, leading to decreased ATP stores and an eventual collapse in energy metabolism [78]. This is due to the ability of AuNPs to alter membrane potential on entry into the cell [78]. Additionally, the creation of ROS is facilitated by AuNPs, further facilitating cell death. The smaller the size of the AuNPs, the greater the surface area and interface for interaction with microbes, demonstrating a stronger antimicrobial effect [79].
While some antibacterial effects are seen, AuNPs are principally used in tissue repair given their anti-inflammatory properties via cytokine modulation and antioxidant properties [80][81]. Substantial antioxidant properties are seen as AuNPs are able to bind free radicals such as nitric oxide (NO) or hydroxyl (OH-) [82][83][84]. This strong catalytic activity in free radical scavenging is further observed through the ability of AuNPs to increase nuclear factor erythroid 2-related factor (NRF2), which allows for antioxidant gene activation [85][86]. Furthermore, while being able to facilitate the creation of ROS, they are also able to receive electrons and remove or deactivate ROS, with greater effects seen the higher the surface area of the AuNPs is [76].
In addition to tissue repair, wound healing is found to be accelerated and ameliorated with the use of AuNPs through the promotion of collagen expression, growth factors, vascular endothelial growth factor (VEGF), fibroblast proliferation, decreased cellular apoptosis, and angiogenesis [20][87].
Despite these beneficial effects, AuNPs must usually be incorporated with other biomolecules for efficacy in wound healing applications. Examples include the incorporation of AuNPs in chitosan or gelatin for the enhancement of wound healing or in collagen for a similar effect [4][5]. One study of a rat full-thickness excisional wound model demonstrated accelerated healing and wound closure with improved hemostasis and re-epithelization compared to the Tegaderm dressing and pure chitosan hydrogel controls in a chitosan-AuNP hydrogel [88]. Recent studies have also incorporated phototherapy in conjunction with AuNPs to achieve antimicrobial activity [6][89].
The summary of all in vivo studies related to AuNP-loaded hydrogels is shown in Table 2.
Table 2. Summary of Characteristics and Findings of Included Trials for Gold Nanoparticles.
| Hydrogel Species | Particle Size (Diameter) | Dosage of NP | Rate of Release | Antimicrobial Capability | Cell Type | Cytotoxic Effect | In Vivo Model | In Vivo Findings | Year | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Cydonia oblonga seed extract | 20–30 nm | 10 mmol | - | MIC: B. simplex: 16 mg/mL; B. subtilis: 32 mg/mL; P. aeruginosa: 32 mg/mL; E. coli: 16 mg/mL; S. aureus: 40 mg/mL; A. niger: 50 mg/mL; P. notatum: 50 mg/mL Inhibition zone: B. Simplex: 15 mm; B. Subtilis: 17 mm; P. aeruginosa: 16 mm; E. Coli: 18 mm; S. Aureus: 12 mm |
- | - | Full-thickness wounds in mice | 99% wound closure in 5 days; increased expression of NANOG and CD-4 in nanoparticle treatment group | 2022 | [90] |
| Alginate | 25 nm (NP), 50, 70, 120 nm spike length (nanostars (NS)) | 1.5 µg/mL | NP: 157 ng release over 12 h; NS: 8.63 ng release over 12 h | The bacterial killing of >95% is observed for P. aeruginosa and E. coli, while up to 60% for Gram-positive S. aureus. >80% of colonies of P. aeruginosa and E. coli were also reduced. 35.4% reduction of colonies were obtained for S. aureus. | NIH-3T3 | 85% viability | S. aureus infected full-thickness wounds in rats | Accelerated wound healing with enhanced wound closure and angiogenesis | 2022 | [91] |
| Poloxamer | 29.2 ± 2.1 nm | - | Slow and prolonged release over 48 h, ~2%/min | High percentage reduction of bacterial viable count against S. aureus and P. aeruginosa | - | - | Full-thickness excisional wound model in rats | Almost completely healed wound after 14 days of daily treatment compared to control, owing to their enhanced skin re-epithelization effect and collagen formation, in addition to their impact on the gene expression of inflammatory and anti-inflammatory mediators. Furthermore, low percentages of deposition into the main body organs after 21 days of daily wound treatment was seen. | 2019 | [92] |
| Polyethyleneimine (PEI), polyethylene glycol (PEG), hexachlorocyclic triphosphonitrile (HCCP) | 22 nm | 0.3, 3, 5 nM | Release ratio of approximately 70% after 16 h incubation with the tendency to slow down thereafter | Improved performance against S. aureus and MRSA, especially if the gel is exposed to laser irradiation | L929 cells | Negligible cytotoxicity, good biocompatibility, and excellent hemocompatibility | Full-thickness excisional wound model in mice | Accelerated wound healing with no toxicity or significant adverse effects compared to the control. | 2022 | [93] |
| Alginate | - | 0.05, 0.1, 0.2 mg/mL | In PBS (pH 7.2–7.4, 0.1 M), approximately 74%, 73%, and 76% of Ga3+ was released from Ga3+-crosslinked hydrogel | Strong bactericidal activity against S. aureus and P. aeruginosa observed with higher reduction in P. aeruginosa compared to S. aureus. Faster release of silver ions demonstrated stronger antibacterial effect. | Keratinocytes (HaCaT) | After 1, 3 and 7 days of incubation, the materials did not show any toxicity even after 7 days of contact and up to 96% of keratinocytes were viable | Full-thickness wounds in diabetic and non-diabetic mice | Rapid contraction of wound edges was seen in comparison to the controls as well as minor scab formation and lack of inflammation in the integument. Fifteen days of treatment with the nano-enabled hydrogels completely recovered the wounds of non-diabetic and diabetic mice. The bactericidal effect was also evidenced by absence of bacterial contamination in the wounds. | 2021 | [94] |
| Chitin | 215.31 nm | 2.5, 5, 10% w/v | - | Hydrogels with Au contents of 5% and 10% were most effective at inhibiting E. coli growth, whereas a content of >2.5% was sufficient to completely inhibit the growth of S. aureus colonies | L929 cells | The survival rate of cells in all concentrations of Au was >80% at 2 h, and even over >90% in most groups. At 48 h, except for the 10% group, the cell viability of other groups was >80%. |
S. aureus infected full-thickness wound model in mice | Good antibacterial, hemostatic, and anti-inflammatory properties. | 2023 | [95] |
2.3. Copper (Cu) and Copper Oxide (CuO)
Previous studies have demonstrated that CuNPs have antimicrobial activity as well as properties that facilitate tissue repair. CuNPs have shown antibacterial activity against bacterial strains such as Escherichia coli and Staphylococcus aureus but also fungicidal effects [96][97][98][99][100][101]. The principal mechanism of action is through adhesion of the CuNP to bacteria due to their opposing electrical charges, resulting in a reduction reaction that weakens and destroys the bacterial cell wall. CuNPs have also shown antibacterial activity through the enhancement of immunity with the promotion of interleukin-2 (IL2) production, as well as its ability to serve as a cofactor for various enzymes such as cytochrome oxidase [102]. Additionally, CuNPs have an influence on cytokine regulation, thus also having anti-inflammatory properties [103]. In terms of tissue repair, ECM synthesis is promoted through the stimulation of ECM components such as fibrinogen and fibroblasts as well as the production of integrins and collagen [104][105]. One study observing the use of a CuNP-embedded hydrogel in the treatment of full-thickness excisional wounds in rats demonstrated an accelerated wound healing rate [5]. Despite these positive effects, CuNPs are prone to rapid oxidation, promotion of the production of free radicals, and instability, thus limiting its use [106][107].
CuO NPs have been used in multiple biomedical settings, such as in drug delivery, as anti-cancer agents, and wound healing given their biocompatibility, low toxicity, and antimicrobial properties [108][109]. The specific mechanism for the antibacterial effects of CuO remains unknown; however, it is postulated that it is related to the generation of ROS within bacterial cells [110]. However, with CuO NPs, antibacterial activity was partially related to bacterial properties. For example, different effects were noted with increased bactericidal activity in Gram-negative organisms, such as E. coli, compared to Gram-positive organisms, such as S. aureus [111]. Despite these antibacterial properties, one concern is toxicity, the induction of oxidative stress, and subsequent DNA and mitochondrial damage [112][113].
2.4. Zinc (Zn) and Zinc Oxide (ZnO)
Zn and ZnO NPs are some of the most commonly used NPs in wound healing applications due to their anti-inflammatory and antimicrobial properties [114]. As inorganic agents, they are more stable than their organic agent counterparts. They are also advantageous in their ability to remain within the wound bed for longer periods of time [114][115]. The antimicrobial effects of Zn and ZnO NPs are due to disruption of cell membranes and oxidant injury [114][115]. Zinc also serves as a cofactor for metalloproteinases and other enzymatic complexes, promoting migration of keratinocytes and regeneration of the ECM [114][115]. A previous study examining full-thickness wounds in a rat model showed accelerated and ameliorated healing compared to control with improved re-epithelialization as well as increased collagen deposition and tissue granulation [115]. Moreover, both Zn and ZnO NPs have demonstrated good biocompatibility and low cytotoxicity [114].
Like other NPs, the Zn and ZnO NP effect is dependent on the size, surface-area-to-volume ratio, and concentration of the NPs [116]. Smaller NPs have been shown to be more cytotoxic given their larger surface-area-to-volume ratio, whereas larger NPs demonstrate increased cytocompatibility [117]. In fact, a previous study demonstrated that ZnO NPs are highly compatible with fibroblast cells and promote their growth, migration, and adhesion [100].
2.5. Other Metal Oxides
Metal oxide NPs include zinc oxide (ZnO), copper oxide (CuO), cerium oxide (CeO2), manganese oxide (MnO2), and titanium oxide (TiO2). These NPs have antioxidant properties and have been shown to facilitate wound healing through the restriction of ROS, inhibiting apoptosis [114][115].
2.5.1. Titanium Oxide (TiO2)
A study of the antimicrobial effects of TiO2 NPs demonstrated little effect but showed accelerated wound healing in a full-thickness excisional wound model [118].
2.5.2. Cerium Oxide (CeO2)
CeO2 NPs have the highest antioxidant activity of all NPs and are most active in the scavenging of free radicals [119]. This is due to the oxygen vacancies of CeO2, leading to the reduction of Cerium from Ce+4 to Ce+3, for example [119].
2.5.3. Manganese Oxide (MnO2)
MnO2 is also a potential candidate to be used in nanoparticle–hydrogel composites. Known to relieve oxidative stress, MnO2 is able to catalytically decompose H2O2 into O2, thus effectively providing a targeted approach to hypoxic relief [120]. One study evaluating MnO2 nanoparticles in the healing of chronic diabetic wounds in vivo demonstrated the eradication of biofilms, attenuation of hyperglycemia, hemostasis, and the creation of an optimized wound environment which reduced inflammation, accelerated granulation tissue formation and re-epithelialization, and accelerated wound healing [120].
2.6. Iron (Fe) Nanoparticles (FeNP)
Less commonly used in antibacterial wound dressing applications, iron nanoparticles have been shown to induce bacterial death, membrane damage, DNA degradation, and lipid peroxidation [121][122][123]. One study demonstrated high antibacterial activities against S. aureus and E. coli both in vitro and in an in vivo infected full-thickness excisional wound model in mice where accelerated wound healing and anti-inflammatory properties were observed [121].
2.7. Gallium (Ga) Nanoparticles (GaNP)
Gallium is very infrequently used in wound healing applications. Given that gallium and iron have equal ionic radii, one study hypothesized that the substitution of iron with gallium would impair bacterial iron metabolism and exert an antimicrobial effect [124]. This has previously been observed in vitro, whereby gallium resulted in reduced bacterial survival [124][125][126]. Moreover, given the inability of gallium to be reduced in physiological environments, a property not shared with iron, gallium also disrupts enzyme activity [127]. A 2022 study by Qin et al. demonstrated the good antimicrobial effect of gallium embedded in an alginate-base hydrogel, with good biocompatibility against NIH3T3 cells in vitro as well as accelerated wound healing with good biocompatibility, angiogenesis, and collagen deposition compared to the control in an S. aureus infected full-thickness excisional wound model in mice [124].
2.8. Combinations of Metal Nanoparticles
Occasionally, metal NPs can be used in conjunction with each other to provide synergistic antibacterial effects. For example, one study investigated the effects of AgNP and CuNP within a chitosan hydrogel, demonstrating good antibacterial activity against S. aureus and E. coli with good biocompatibility and accelerated healing compared to control in an S. aureus infected full-thickness excisional wound model in type 1 diabetic rats [128]. Another study looked at the synergy between ZnO and AgNPs, demonstrating an excellent bactericidal effect against E. coli and S. aureus [129]. Interestingly, AgNPs were observed to exhibit a small amount of cytotoxicity when tested against mouse calvarial (MC3T3-E1) cells alone, but when used in conjunction with ZnO NPs, lower cytotoxicity was seen [129]. In an S. aureus infected partial thickness wound model in rats, the release of Ag+ and Zn2+ was found to stimulate immune function to produce a large number of white blood cells and neutrophils (2–4 times more than the control), thereby producing the synergistic antibacterial effects and accelerated wound healing [130].
This entry is adapted from the peer-reviewed paper 10.3390/gels9070591
References
- Definition of Nanotechnology. Available online: https://www.merriam-webster.com/dictionary/nanotechnology (accessed on 11 January 2023).
- Nanotechnology in Medicine. Available online: https://onlinelibrary.wiley.com/doi/epub/10.1002/9781119769897 (accessed on 11 January 2023).
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Cliu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136.
- Chen, S.-A.; Chen, H.-M.; Yao, Y.-D.; Hung, C.-F.; Tu, C.-S.; Liang, Y.-J. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012, 47, 875–883.
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Rao, J.V. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237.
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869.
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612.
- Barroso, A.; Mestre, H.; Ascenso, A.; Simões, S.; Reis, C. Nanomaterials in wound healing: From material sciences to wound healing applications. Nano Sel. 2020, 1, 443–460.
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306.
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA delivery through nanoparticles. J. Control. Release 2019, 313, 80–95.
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171.
- Yates, C.C.; Hebda, P.; Wells, A. Skin Wound Healing and Scarring: Fetal Wounds and Regenerative Restitution. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 325–333.
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care—Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390.
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of Silver in the Prevention and Treatment of Infections: Silver Review. Surg. Infect. 2013, 14, 8–20.
- Cameron, P.; Gaiser, B.K.; Bhandari, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431–437.
- Navani, N.K.; Lambadi, P.R.; Sharma, T.K.; Kumar, P.; Vasnani, P.; Thalluri, S.M.; Bisht, N.; Pathania, R. Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int. J. Nanomed. 2015, 10, 2155–2171.
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, 13719.
- Guide to the Quality and Safety of Tissues and Cells for Human Application—European Directorate for the Quality of Medi-cines & HealthCare—EDQM. European Directorate for the Quality of Medicines & HealthCare. Available online: https://www.edqm.eu/en/guide-to-the-quality-and-safety-of-tissues-and-cells-for-human-application1 (accessed on 20 January 2023).
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591.
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juránová, J.; Ulrichova, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, e137–e142.
- Wong, K.K.Y.; Cheung, S.O.F.; Huang, L.; Niu, J.; Tao, C.; Ho, C.-M.; Che, C.-M.; Tam, P.K.H. Further Evidence of the Anti-inflammatory Effects of Silver Nanoparticles. Chemmedchem 2009, 4, 1129–1135.
- Lara, H.H.; Ayala-Núñez, N.V.; Turrent, L.D.C.I.; Padilla, C.R. Bactericidal Effect of Silver Nanoparticles against Multidrug-Resistant Bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621.
- Kreytsberg, G.N.; Gracheva, I.E.; Kibrik, B.S.; Golikov, I.V. Antituberculous effect of silver nanoparticles. J. Phys. Conf. Ser. 2011, 291, 012030.
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551.
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588.
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590.
- Liu, X.; Lee, P.-Y.; Ho, C.-M.; Lui, V.C.H.; Chen, Y.; Che, C.-M.; Tam, P.K.H.; Wong, K.K.Y. Silver Nanoparticles Mediate Differential Responses in Keratinocytes and Fibroblasts during Skin Wound Healing. Chemmedchem 2010, 5, 468–475.
- GhavamiNejad, A.; Unnithan, A.R.; Sasikala, A.R.K.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Park, C.H.; et al. Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183.
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913.
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Central Sci. 2017, 3, 163–175.
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308.
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166.
- Bharathi, S.; Ramesh, B.; Kumaran, S.; Radhakrishnan, M.; Saravanan, D.; Pugazhvendan, S.R.; Nalinasundari, M.S. Development of nanobiomaterial for wound healing based on silver nanoparticles loaded on chitosan hydrogel. 3 Biotech 2021, 11, 490.
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lapmanee, S.; Kulchat, S. Enhanced wound healing properties of guar gum/curcumin-stabilized silver nanoparticle hydrogels. Sci. Rep. 2021, 11, 21836.
- Chen, Q.; Li, S.; Zhao, W.; Zhao, C. A rapid-triggered approach towards antibacterial hydrogel wound dressing with synergic photothermal and sterilization profiles. Biomater. Adv. 2022, 138, 212873.
- Lin, Z.; Fan, D.; Li, G.; He, L.; Qin, X.; Zhao, B.; Wang, Q.; Liang, W. Antibacterial, Adhesive, and Conductive Hydrogel for Diabetic Wound Healing. Macromol. Biosci. 2022, 23, 2200349.
- Sharifi, E.; Sadati, S.A.; Yousefiasl, S.; Sartorius, R.; Zafari, M.; Rezakhani, L.; Alizadeh, M.; Zare, E.N.; Omidghaemi, S.; Ghanavatinejad, F.; et al. Cell loaded hydrogel containing Ag-doped bioactive glass–ceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration. Bioeng. Transl. Med. 2022, 7, e10386.
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles From Azadirachta indica. Front. Microbiol. 2021, 12, 611560.
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar free healing of full thickness diabetic wounds: A unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix. Int. J. Biol. Macromol. 2021, 178, 41–52.
- De Lima, G.G.; De Lima, D.W.F.; De Oliveira, M.J.A.; Lugão, A.B.; Alcântara, M.T.S.; Devine, D.M.; De Sá, M.J.C. Synthesis and in Vivo Behavior of PVP/CMC/Agar Hydrogel Membranes Impregnated with Silver Nanoparticles for Wound Healing Applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852.
- Cao, C.; Yang, N.; Zhao, Y.; Yang, D.; Hu, Y.; Yang, D.; Song, X.; Wang, W.; Dong, X. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today 2021, 39, 101165.
- Deng, P.; Chen, F.; Zhang, H.; Chen, Y.; Zhou, J. Conductive, Self-Healing, Adhesive, and Antibacterial Hydrogels Based on Lignin/Cellulose for Rapid MRSA-Infected Wound Repairing. ACS Appl. Mater. Interfaces 2021, 13, 52333–52345.
- Diniz, F.R.; Maia, R.C.A.P.; Andrade, L.R.; Andrade, L.N.; Chaud, M.V.; Da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Da Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390.
- Garg, S.; Chandra, A.; Mazumder, A.; Mazumder, R. Green synthesis of silver nanoparticles using Arnebia nobilis root extract and wound healing potential of its hydrogel. Asian J. Pharm. 2014, 8, 95.
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223.
- Haidari, H.; Bright, R.; Strudwick, X.L.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds. Acta Biomater. 2021, 128, 420–434.
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157.
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641.
- Lee, Y.-H.; Hong, Y.-L.; Wu, T.-L. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111385.
- Li, B.; Li, H.; Yang, H.; Shu, Y.; Li, K.; Chen, K.; Xiao, W.; Liao, X. Preparation and antibacterial properties of an /GelMA composite hydrogel. Biomed. Mater. 2022, 17, 025005.
- Liu, K.; Dai, L.; Li, C. A lignocellulose-based nanocomposite hydrogel with pH-sensitive and potent antibacterial activity for wound healing. Int. J. Biol. Macromol. 2021, 191, 1249–1254.
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70.
- Liu, Y.; Fan, J.; Lv, M.; She, K.; Sun, J.; Lu, Q.; Han, C.; Ding, S.; Zhao, S.; Wang, G.; et al. Photocrosslinking silver nanoparticles–aloe vera–silk fibroin composite hydrogel for treatment of full-thickness cutaneous wounds. Regen. Biomater. 2021, 8, rbab048.
- Lustosa, A.K.M.F.; Oliveira, A.C.D.J.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.D.G.N.; Delerue-Matos, C.; Oliveira, R.D.C.M.D.; et al. In Situ Synthesis of Silver Nanoparticles in a Hydrogel of Carboxymethyl Cellulose with Phthalated-Cashew Gum as a Promising Antibacterial and Healing Agent. Int. J. Mol. Sci. 2017, 18, 2399.
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An injectable metal nanoparticle containing cellulose derivative-based hydrogels: Evaluation of antibacterial and in vitro-vivo wound healing activity in children with burn injuries. Int. Wound J. 2021, 19, 666–678.
- Mei, J.; Zhou, J.; Kong, L.; Dai, Y.; Zhang, X.; Song, W.; Zhu, C. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J. Nanobiotechnol. 2022, 20, 232.
- Martínez-Higuera, A.; Rodríguez-Beas, C.; Villalobos-Noriega, J.M.A.; Arizmendi-Grijalva, A.; Ochoa-Sánchez, C.; Larios-Rodríguez, E.; Martínez-Soto, J.M.; Rodríguez-León, E.; Ibarra-Zazueta, C.; Mora-Monroy, R.; et al. Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci. Rep. 2021, 11, 11312.
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36.
- Mugade, M.; Patole, M.; Pokharkar, V. Bioengineered mannan sulphate capped silver nanoparticles for accelerated and targeted wound healing: Physicochemical and biological investigations. Biomed. Pharmacother. 2017, 91, 95–110.
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2018, 35, 1837–1845.
- Hu, J.; Tao, M.; Sun, F.; Chen, C.; Chen, G.; Wang, G. Multifunctional hydrogel based on dopamine-modified hyaluronic acid, gelatin and silver nanoparticles for promoting abdominal wall defect repair. Int. J. Biol. Macromol. 2022, 222, 55–64.
- Ren, Y.; Ailierken, A.; Zhao, L.; Lin, Z.; Jiang, J.; Li, B.; Wang, J.; Hua, J.; Tu, Q. hUC-MSCs lyophilized powder loaded polysaccharide ulvan driven functional hydrogel for chronic diabetic wound healing. Carbohydr. Polym. 2022, 288, 119404.
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Mussel-inspired adhesive bilayer hydrogels for bacteria-infected wound healing via NIR-enhanced nanozyme therapy. Colloids Surfaces B Biointerfaces 2021, 210, 112230.
- Kong, F.; Fan, C.; Yang, Y.; Lee, B.H.; Wei, K. 5-hydroxymethylfurfural-embedded poly (vinyl alcohol)/sodium alginate hybrid hydrogels accelerate wound healing. Int. J. Biol. Macromol. 2019, 138, 933–949.
- He, H.; Xia, D.-L.; Chen, Y.-P.; Li, X.-D.; Chen, C.; Wang, Y.-F.; Shen, L.; Hu, Y.-L.; Gu, H.-Y. Evaluation of a two-stage antibacterial hydrogel dressing for healing in an infected diabetic wound: ANTIBACTERIAL HYDROGEL DRESSING FOR DIABETIC WOUND HEALING. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1808–1817.
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171.
- Tang, Q.; Chen, C.; Jiang, Y.; Huang, J.; Liu, Y.; Nthumba, P.M.; Gu, G.; Wu, X.; Zhao, Y.; Ren, J. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J. Mater. Chem. B 2020, 8, 5756–5764.
- Deng, M.; Wu, Y.; Ren, Y.; Song, H.; Zheng, L.; Lin, G.; Wen, X.; Tao, Y.; Kong, Q.; Wang, Y. Clickable and smart drug delivery vehicles accelerate the healing of infected diabetic wounds. J. Control. Release 2022, 350, 613–629.
- Xiao, L.; Hui, F.; Tian, T.; Yan, R.; Xin, J.; Zhao, X.; Jiang, Y.; Zhang, Z.; Kuang, Y.; Li, N.; et al. A Novel Conductive Antibacterial Nanocomposite Hydrogel Dressing for Healing of Severely Infected Wounds. Front. Chem. 2021, 9, 787886.
- Du, T.; Xiao, Z.; Cao, J.; Wei, L.; Li, C.; Jiao, J.; Song, Z.; Liu, J.; Du, X.; Wang, S. NIR-activated multi-hit therapeutic Ag2S quantum dot-based hydrogel for healing of bacteria-infected wounds. Acta Biomater. 2022, 145, 88–105.
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084.
- Zhao, F.; Liu, Y.; Song, T.; Zhang, B.; Li, D.; Xiao, Y.; Zhang, X. A chitosan-based multifunctional hydrogel containing in situ rapidly bioreduced silver nanoparticles for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 2135–2147.
- Xiong, Y.; Xu, Y.; Zhou, F.; Hu, Y.; Zhao, J.; Liu, Z.; Zhai, Q.; Qi, S.; Zhang, Z.; Chen, L. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng. Transl. Med. 2022, 8, e10373.
- Xiang, J.; Bai, Y.; Huang, Y.; Lang, S.; Li, J.; Ji, Y.; Peng, B.; Liu, G. A zwitterionic silver nanoparticle-incorporating injectable hydrogel with a durable and efficient antibacterial effect for accelerated wound healing. J. Mater. Chem. B 2022, 10, 7979–7994.
- Guo, R.; Song, Y.; Wang, G.; Murray, R.W. Does Core Size Matter in the Kinetics of Ligand Exchanges of Monolayer-Protected Au Clusters? J. Am. Chem. Soc. 2005, 127, 2752–2757.
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.S.; Eom, S.; Gurunathan, S. RAesneatrcih-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16.
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315.
- Zain, N.M.; Stapley, A.; Shama, G. Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications. Carbohydr. Polym. 2014, 112, 195–202.
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; Silveira, G.D.B.; de Souza, D.L.; Brandolfi, J.D.A.; de Souza, C.T.; Paula, M.M.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C 2017, 77, 476–483.
- Li, X.; Wang, H.; Rong, H.; Li, W.; Luo, Y.; Tian, K.; Quan, D.; Wang, Y.; Jiang, L. Effect of composite SiO 2 @AuNPs on wound healing: In vitro and vivo studies. J. Colloid Interface Sci. 2015, 445, 312–319.
- Victor, E.G.; Silveira, P.C.; Possato, J.C.; da Rosa, G.L.; Munari, U.B.; de Souza, C.T.; Pinho, R.A.; da Silva, L.; Streck, E.L.; Paula, M.M. Pulsed ultrasound associated with gold nanoparticle gel reduces oxidative stress parameters and expression of pro-inflammatory molecules in an animal model of muscle injury. J. Nanobiotechnol. 2012, 10, 11.
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161.
- Leu, J.-G.; Chen, S.-A.; Chen, H.-M.; Wu, W.-M.; Hung, C.-F.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 767–775.
- Esumi, K.; Houdatsu, H.; Yoshimura, T. Antioxidant Action by Gold−PAMAM Dendrimer Nanocomposites. Langmuir 2004, 20, 2536–2538.
- Rattanata, N.; Daduang, S.; Wongwattanakul, M.; Leelayuwat, C.; Limpaiboon, T.; Lekphrom, R.; Sandee, A.; Boonsiri, P.; Chio-Srichan, S.; Daduang, J. Gold Nanoparticles Enhance the Anticancer Activity of Gallic Acid against Cholangiocarcinoma Cell Lines. Asian Pac. J. Cancer Prev. 2015, 16, 7143–7147.
- Cheng, H.; Lai, G.; Fu, L.; Zhang, H.; Yu, A. Enzymatically catalytic deposition of gold nanoparticles by glucose oxidase-functionalized gold nanoprobe for ultrasensitive electrochemical immunoassay. Biosens. Bioelectron. 2015, 71, 353–358.
- Haupenthal, D.P.D.S.; Mendes, C.; Silveira, G.D.B.; Zaccaron, R.P.; Corrêa, M.E.A.B.; Nesi, R.T.; Pinho, R.; Paula, M.M.D.S.; Silveira, P.C.L. Effects of treatment with gold nanoparticles in a model of acute pulmonary inflammation induced by lipopolysaccharide. J. Biomed. Mater. Res. Part A 2019, 108, 103–115.
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970.
- Tuhin, R.H.; Begum, M.M.; Rahman, S.; Karim, R.; Begum, T.; Ahmed, S.U.; Mostofa, R.; Hossain, A.; Abdel-Daim, M.; Begum, R. Wound healing effect of Euphorbia hirta linn. (Euphorbiaceae) in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 423.
- Batool, Z.; Muhammad, G.; Iqbal, M.M.; Aslam, M.S.; Raza, M.A.; Sajjad, N.; Abdullah, M.; Akhtar, N.; Syed, A.; Elgorban, A.M.; et al. Hydrogel assisted synthesis of gold nanoparticles with enhanced microbicidal and in vivo wound healing potential. Sci. Rep. 2022, 12, 6575.
- Kaul, S.; Sagar, P.; Gupta, R.; Garg, P.; Priyadarshi, N.; Singhal, N.K. Mechanobactericidal, Gold Nanostar Hydrogel-Based Bandage for Bacteria-Infected Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 44084–44097.
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186.
- Shang, K.; Tao, L.; Jiang, S.; Yan, J.; Hu, S.; Yang, G.; Ma, C.; Cheng, S.; Wang, X.; Yin, J. Highly flexible hydrogel dressing with efficient antibacterial, antioxidative, and wound healing performances. Biomater. Sci. 2022, 10, 1373–1383.
- Pérez-Rafael, S.; Ivanova, K.; Stefanov, I.; Puiggalí, J.; del Valle, L.J.; Todorova, K.; Dimitrov, P.; Hinojosa-Caballero, D.; Tzanov, T. Nanoparticle-driven self-assembling injectable hydrogels provide a multi-factorial approach for chronic wound treatment. Acta Biomater. 2021, 134, 131–143.
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact. Mater. 2023, 20, 355–367.
- Melamed, E.; Kiambi, P.; Okoth, D.; Honigber, I.; Tamir, E.; Borkow, G. Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings—Case Series. Medicina 2021, 57, 296.
- Baek, J.H.; Yoo, M.A.; Koh, J.S.; Borkow, G. Reduction of facial wrinkles depth by sleeping on copper oxide-containing pillowcases: A double blind, placebo controlled, parallel, randomized clinical study: Wrinkles reduction with copper pillowcases. J. Cosmet. Dermatol. 2012, 11, 193–200.
- Dykes, P. Increase in skin surface elasticity in normal volunteer subjects following the use of copper oxide impregnated socks. Ski. Res. Technol. 2014, 21, 272–277.
- Barani, M.; Rahdar, A.; Mukhtar, M.; Razzaq, S.; Qindeel, M.; Olam, S.A.H.; Paiva-Santos, A.C.; Ajalli, N.; Sargazi, S.; Balakrishnan, D.; et al. Recent application of cobalt ferrite nanoparticles as a theranostic agent. Mater. Today Chem. 2022, 26, 101131.
- Manuja, A.; Raguvaran, R.; Kumar, B.; Kalia, A.; Tripathi, B. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020, 155, 823–833.
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.-A.; Mohamed, M.A. Reduced Graphene Oxide Nanosheet-Decorated Copper Oxide Nanoparticles: A Potent Antifungal Nanocomposite against Fusarium Root Rot and Wilt Diseases of Tomato and Pepper Plants. Nanomaterials 2020, 10, 1001.
- Hopkins, R.G.; Failla, M.L. Copper Deficiency Reduces Interleukin-2 (IL-2) Production and IL-2 mRNA in Human T-Lymphocytes. J. Nutr. 1997, 127, 257–262.
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The neglected role of copper ions in wound healing. J. Inorg. Biochem. 2016, 161, 1–8.
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, S952–S959.
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.I.; Lavie, Y.; Grunfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.C.; Marikovsky, M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010, 18, 266–275.
- Quaranta, D.; Krans, T.; Santo, C.E.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of Contact-Mediated Killing of Yeast Cells on Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426.
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116.
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjugate Chem. 2016, 27, 1188–1199.
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75.
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2013, 60, 208–337.
- Ungur, G.; Hruza, J. Influence of copper oxide on the formation of polyurethane nanofibers via electrospinning. Fibers Polym. 2015, 16, 621–628.
- Xiao, Y.; Reis, L.A.; Feric, N.; Knee, E.J.; Gu, J.; Cao, S.; Laschinger, C.; Londono, C.; Antolovich, J.; McGuigan, A.P.; et al. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl. Acad. Sci. USA 2016, 113, E5792–E5801.
- Marrazzo, P.; O’leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104.
- Jaiswal, M.; Koul, V.; Dinda, A.K. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with a silver-coated chitosan wafer for full-thickness skin wounds. J. Appl. Polym. Sci. 2016, 133, 21.
- Yadid, M.; Feiner, R.; Dvir, T. Gold Nanoparticle-Integrated Scaffolds for Tissue Engineering and Regenerative Medicine. Nano Lett. 2019, 19, 2198–2206.
- Czyżowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles–friends or enemies? Int. J. Environ. Health Res. 2020, 32, 885–901.
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177.
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57.
- Zhang, F.; Chan, S.-W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129.
- Wang, Q.; Qiu, W.; Li, M.; Li, N.; Li, X.; Qin, X.; Wang, X.; Yu, J.; Li, F.; Huang, L.; et al. Mussel-inspired multifunctional hydrogel dressing with hemostasis, hypoglycemic, photothermal antibacterial properties on diabetic wounds. Biomater. Sci. 2022, 10, 4796–4814.
- Xu, Z.; Gao, Z.; Lu, J.; Wang, T.; Wang, W.; Fan, L.; Xi, J.; Han, B. Ferrous iron-induced formation of glycyrrhizic acid hydrogels for Staphylococcus aureus-infected wound healing. Colloids Surf. B Biointerfaces 2023, 221, 112977.
- Xi, J.; An, L.; Huang, Y.; Jiang, J.; Wang, Y.; Wei, G.; Xu, Z.; Fan, L.; Gao, L. Ultrasmall FeS2 Nanoparticles-Decorated Carbon Spheres with Laser-Mediated Ferrous Ion Release for Antibacterial Therapy. Small 2021, 17, e2005473.
- Shen, X.; Ma, R.; Huang, Y.; Chen, L.; Xu, Z.; Li, D.; Meng, X.; Fan, K.; Xi, J.; Yan, X.; et al. Nano-decocted ferrous polysulfide coordinates ferroptosis-like death in bacteria for anti-infection therapy. Nano Today 2020, 35, 100981.
- Qin, J.; Li, M.; Yuan, M.; Shi, X.; Song, J.; He, Y.; Mao, H.; Kong, D.; Gu, Z. Gallium(III)-Mediated Dual-Cross-Linked Alginate Hydrogels with Antibacterial Properties for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 22426–22442.
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520.
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384.
- Choi, S.-R.; Switzer, B.; Britigan, B.E.; Narayanasamy, P. Gallium Porphyrin and Gallium Nitrate Synergistically Inhibit Mycobacterial Species by Targeting Different Aspects of Iron/Heme Metabolism. ACS Infect. Dis. 2020, 6, 2582–2591.
- Liu, X.; Zhou, S.; Cai, B.; Wang, Y.; Deng, D.; Wang, X. An injectable and self-healing hydrogel with antibacterial and angiogenic properties for diabetic wound healing. Biomater. Sci. 2022, 10, 3480–3492.
- Zhang, Y.; Liu, X.; Li, Z.; Zhu, S.; Yuan, X.; Cui, Z.; Yang, X.; Chu, P.K.; Wu, S. Nano Ag/ZnO-Incorporated Hydroxyapatite Composite Coatings: Highly Effective Infection Prevention and Excellent Osteointegration. ACS Appl. Mater. Interfaces 2018, 10, 1266–1277.
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag//ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021.
This entry is offline, you can click here to edit this entry!
