Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Urology & Nephrology
Benign prostatic hyperplasia (BPH) is a chronic proliferative disease showing stromal-dominant proliferation. Inflammation in BPH tissues by various factors finally leads to tissue remodeling and stromal proliferation through the wound healing process of the prostate. The stromal proliferation of BPH develops by two pathways, including androgen-dependent and androgen-independent pathways.
- benign prostatic hyperplasia
- stromal proliferation
1. Introduction
Benign prostatic hyperplasia (BPH) is a chronic proliferative disease that may be defined as prostate glandular epithelial enlargement secondary to epithelial and stromal hyperproliferation, with a predominance of stromal cells [1]. BPH is a progressive condition of aging men that McNeal characterized as a selective benign overgrowth within the transition-periurethral zone (TPZ) proximal to the verumontanum, which can clinically result in bladder outlet obstruction and various lower urinary tract symptoms (LUTS) [2,3,4,5,6]. Beginning at age 40 years, the prevalence of BPH increases with aging. Approximately fifty percent of males can exhibit BPH symptoms by 51–60 years [7]. The prevalence of BPH was seventy percent in males by 70 years, and increases to eighty percent by 85 years [8]. The occurrence and development of BPH are closely related to age.
The human prostatic gland is composed of secretory epithelium arranged in the gland within a fibromuscular stroma composed of smooth muscle. Understanding prostate development clarifies some of the hyperplastic changes observed in BPH, since this disease has been seen historically as a type of embryonic reawakening process [4,9]. Prostatic embryonic development, subsequent pubertal and adult growth, and homeostatic maintenance are strongly regulated through androgen-dependent reciprocal paracrine interactions between stromal and epithelial components (stromal-epithelial interaction) [10]. Cell proliferation is greatly increased in BPH compared to normal prostate tissue; epithelial proliferation was 9-fold higher, while stromal proliferation was 37-fold higher [11], suggesting that BPH is a disease that has stromal-dominant proliferation. Prostate epithelial proliferation results in enlargement of glandular nodules, while stromal proliferation produces a more diffuse hyperplasia with increased extracellular matrix production including collagen type I [12]. However, the specific mechanisms that promote prostatic enlargement, as well as the pathological changes leading to the BPH phenotype, have remained unclear.
Aging and androgens are necessary for the development of BPH, but the pathogenesis of BPH is still largely unresolved [13]. Although androgen levels in men generally decrease with age, there is a paradox that prostate weight increases in BPH patients [14]. On the other hand, clinical practice has proven that a 5α-reductase inhibitor (5AR-I) can effectively reduce the level of dihydrotestosterone (DHT) in prostate tissue and reduce the risk of BPH progression, but 10% of patients still have clinical progression [15,16]. This shows that androgens are not the only factors that cause BPH. Androgen-independent factors include ischemia, oxidative stress, metabolic syndrome, infection, autoimmune reactions, and inflammation (Figure 1), and these interact with each other to form more complicated pathophysiology. In particular, inflammation has received much attention, and various studies are underway. Therefore, to elucidate the pathogenesis of BPH, an approach that focuses on androgen-independent factors is required.
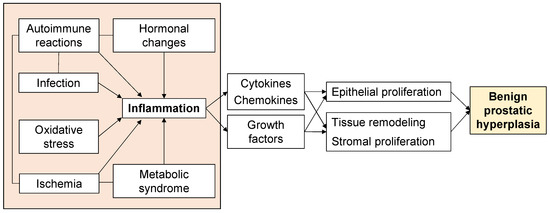
Figure 1. Factors associated with the proliferative process of BPH.
2. Histological Features of Benign Prostatic Hyperplasia
BPH is characterized as a chronic, progressive, but discontinuous hyperplasia of both glandular epithelial and stromal components leading to prostatic enlargement and clinical symptoms [9,17]. Franks and McNeal emphasized the idea that BPH is one of the nodular diseases, and that BPH nodules have several types including stromal, fibromuscular, muscular, fibroadenomatous, and fibromyoadenomatous [9,18]. The stromal components of BPH also contain fibroblasts, myofibroblasts, blood vessels, nerves, and inflammatory cells. Each of these epithelial and stromal components have the possibility of being involved in the development of BPH. Histological BPH can be defined as epithelial and stromal proliferation in the prostate transition zone associated with tissue remodeling, which involves epithelial tissue and fibromuscular matrix, with a certain androgen dependence [19,20,21]. Compared with normal prostatic tissue, the balance between growth and apoptosis of stromal cells in hyperplastic nodules is lost, finally leading to an increase in stromal volume.
McNeal also reported that the adult stromal nodule had re-acquired embryonic activity and was inducing the formation of a glandular duct that was invading into the nearby stromal nodule. This concept was based on the demonstration that embryonic prostatic development is induced by urogenital sinus mesenchyme (UGM), as the embryonic reawakening theory [22].
3. Stromal-Epithelial Interaction in Benign Prostatic Hyperplasia
The effect of UGM on prostatic development is one of the most basic developmental mechanisms, namely the stromal-epithelial interaction. The organs of the male and female urogenital tracts develop through stromal-epithelial interactions that are in sequence regulated by steroid hormones acting through mesenchymal and epithelial steroid receptors [23]. The importance of the stromal component in prostate proliferation has been described. In classic tissue recombination experiments, androgen signaling was reported to be required in the stroma, although it is dispensable in the epithelium for the induction of prostatic tissues [24]. In addition, mosaic ablation of the transforming growth factor (TGF) β type II receptor in a subset of fibroblasts was clarified to result in epithelial neoplasia [25,26]. Moreover, overexpression of fibroblast growth factor 10 by UGM promotes the growth of cancer in adult dissociated prostate epithelial cells grafted under the kidney capsule [27]. There are the interactions between the UGM and urogenital epithelia (UGE) in proliferative process of prostate. The UGM specifies prostatic epithelial identity and induces epithelial invading and budding, and the developing prostatic epithelium likewise induces smooth muscle differentiation and developing a pattern of UGM [28,29]. In transplantation experiments in which UGM alone is transplanted under the kidney capsule of male nude mice, a small amount of smooth muscle differentiated in the grafts [29]. On the other hand, tissue recombinants, indicating a mixture of two cells to evaluate their interaction with each other, composed of UGM and UGE induces the formation of prostatic ducts with epithelial cells surrounded by smooth muscle cells [30]. Importantly, smooth muscle cells can be specified in the UGM not only by UGE, but also by epithelium from adult prostate, presenting common inductive signals across epithelial types and stages [31]. The signaling by interaction between the epithelium and the stroma is not only crucial for prostate development, but it also continues to play key roles in maintaining prostate homeostasis in the adult prostate. These results demonstrate the importance of the stroma and stromal-epithelial interaction in the BPH proliferative process.
Several studies have reported the effectiveness of treatments targeting the stromal-epithelial interaction in BPH. One current study aims to elucidate the potential roles of bone morphogenic protein (BMP) 5 and correlated signaling pathways for the epithelial-mesenchymal transition (EMT) in BPH. This study reported that BMP5 was upregulated in human and rat hyperplastic prostate tissues and localized both in the epithelial and stromal area of prostate tissues. Overexpression of BMP5 enhanced cell proliferation and the EMT process through phosphorylation of small mother against decapentaplegic (Smad) 1/5/8, while selective knockdown of BMP5 induced cell cycle arrest at the G0/G1 phase and inhibited the EMT process. Moreover, the agonist and antagonist of BMP/Smad signaling pathway inverted the effects of BMP5 silence and overexpression, describing that BMP5 regulated cell proliferation and the EMT process through the BMP/Smad signaling pathway, which might contribute to the development of BPH [32].
Hedgehog (HH) signaling is reported to be a master regulator in numerous developmental processes [33]. HH signaling regulates ductal morphogenesis through stromal-epithelial interactions during prostate development [33]. In the adult prostate, HH signaling is active in the stromal component, and HH-responding cells show properties of stromal stem cells. The roles of HH signaling in prostate development have been previously reported by the Bushman group and others [34,35,36].
4. Stromal Proliferation in Benign Prostatic Hyperplasia
The stromal proliferation of BPH develops by two pathways, including androgen-dependent and androgen-independent pathways. The role of androgen in BPH development has been definitive. One study reported increased migration of macrophages and proliferation of prostate stromal cells in a co-culture trans well system. This result showed that targeting the androgen receptor (AR) via an AR degradation enhancer, ASC-J9®, or neutralization of CC-chemokine ligand (CCL) 3 with an antibody, resulted in suppression of macrophage migration and prostate stromal cell proliferation [37]. In previous research [14], the changes of androgen levels and tissue remodeling by aging are generally considered to be the main determinant factor of BPH. In prostate epithelial and stromal cells, testosterone produced by the testis spreads into the prostate epithelium and stromal cells. In stromal cells, most testosterone is changed to DHT, which can work in an autocrine manner in stromal cells or spread into nearby epithelial cells, working in a paracrine manner [38]. It has a high affinity for the AR [21,38,39], which can be regulated by the activated several growth factors or their receptors. Therefore, AR expression influences cell proliferation in both epithelial and stromal cells [40]. However, the role of androgen-independent factors in the proliferative process of BPH is also important as described above. Thus, BPH is a multifactorial disease, which further complicates the pathophysiology of BPH.
Several BPH rat models were developed to evaluate the stromal proliferation of BPH. Kamijo et al. reported a rat model of non-bacterial prostatitis as an animal model with the abundant stromal components [41]. However, this rat model which is produced by administering estradiol after castration has more epithelial than stromal components and was not similar to human BPH. On the other hand, Mori et al. developed the stromal-dominant BPH rat model with the urogenital sinus isolated from male rat 20-day embryos implanted into pubertal male rat ventral prostates, based on the “embryonic reawakening theory” [42]. Histological findings demonstrated that the ratio of stromal to total area was approximately 70%. This BPH rat model is characterized by the proliferation of both epithelial and stromal components with stromal-dominant, similar to human BPH tissues histologically, and it might be a suitable model for evaluating the stromal proliferation of BPH (Figure 2) [42,43]. Using this BPH model, whole-genome oligonucleotide microarray analysis of prostate specimens during the BPH proliferative process was carried out [44]. Gene ontology analysis showed that there were significant changes in gene expression associated with infection, wound healing, and the inflammatory response in BPH tissues. Functional network and pathway analyses showed that genes associated with apoptosis modulation by heat shock protein 70, interleukin (IL)-1, IL-2 and IL-5 signaling pathways, KIT signaling pathway, and secretin-like G-protein-coupled receptors class B, were relatively activated during the proliferative process in BPH tissues. In addition, the expression of genes related to the classical complement pathway and the immune response-related pathway is upregulated, suggesting the possibility that activation of the immune response is involved during the BPH proliferative process. Although these identified genes are associated with both epithelial and stromal proliferation in BPH, these may be more involved in stromal proliferation considering the histological features of this rat model.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241411634
This entry is offline, you can click here to edit this entry!
