The oceans represent the largest ecosystem on earth, with a high diversity of organisms. Oceans have received some attention, and promising compounds with antimicrobial activities were isolated from marine organisms such as bacteria, fungi, algae, sea cucumbers, sea sponges, etc.
- marine compounds
- antibacterial activity
- bacterial resistance
1. Antifungal Compounds Isolated from Marine Bacteria
|
Isolated Compound |
Marine Sources |
Activities |
MIC µg/mL |
|---|---|---|---|
|
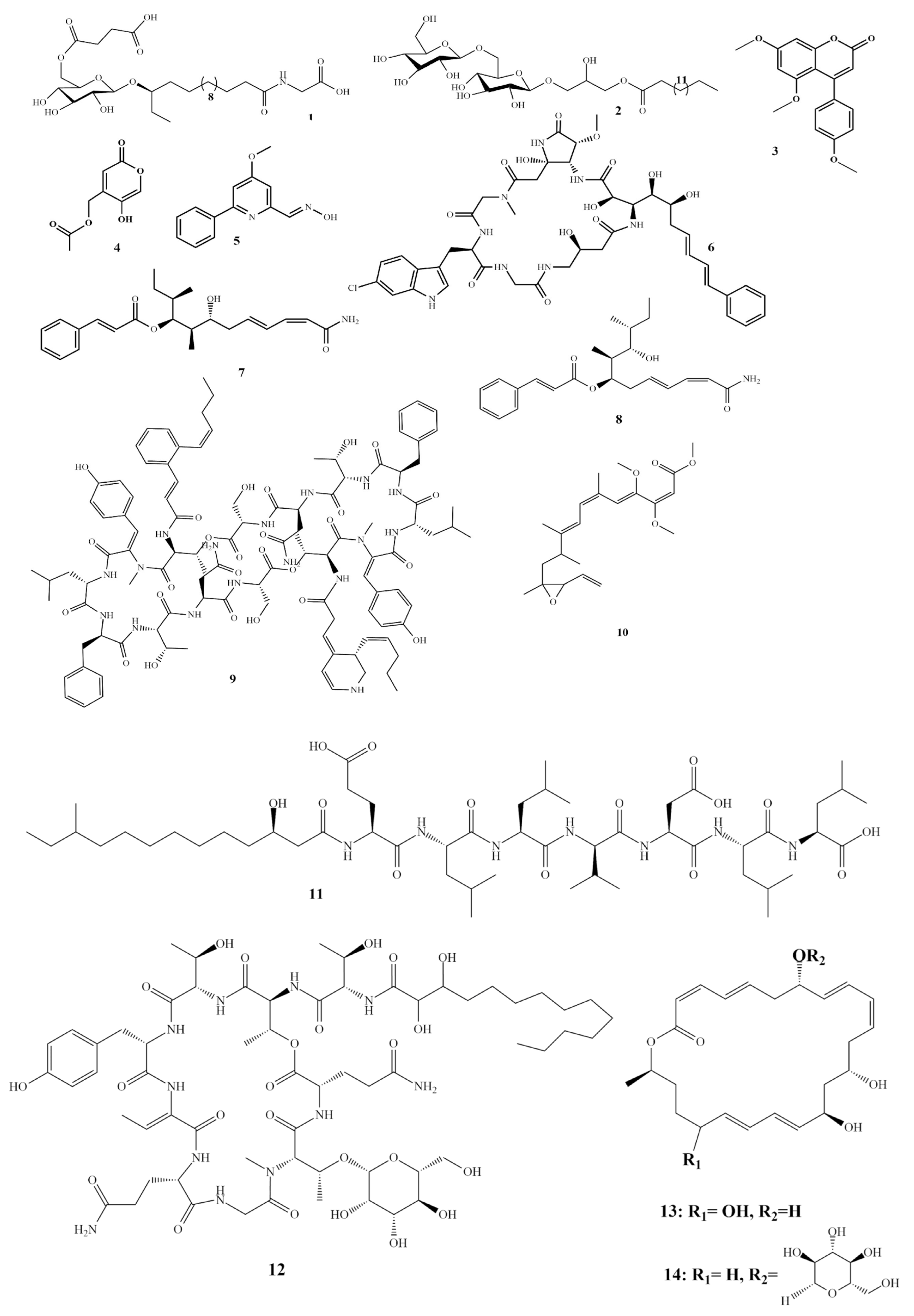
Basiliskamides A (7) Basiliskamides B (8) [7] |
Bacillus laterosporus |
C. albicans A. fumigatus |
1.0–5.0 |
|
Mohangamide A (9) [8] |
Streptomyces sp. |
C. albicans |
4.14 |
|
Haliangicin (10) |
Haliangium luteum |
Botrytis cinerea Pythium ultimum Saprolegnia parasitica |
3.1 0.4 0.1 |
|
Gageostatin A (11) |
B. subtilis 109GGC020 |
Rhizoctonia solani B. cinerea |
4 |
|
Hassallidin A (12) [13] |
epilithic cyanobacteria |
A. fumigatus C. albicans |
4.8 |
|
Macrolactins T (13) Macrolactins B (14) [14] |
Bufo marinus |
Pyricularia oryzae Alternaria. solani |
0.8–4.8 |
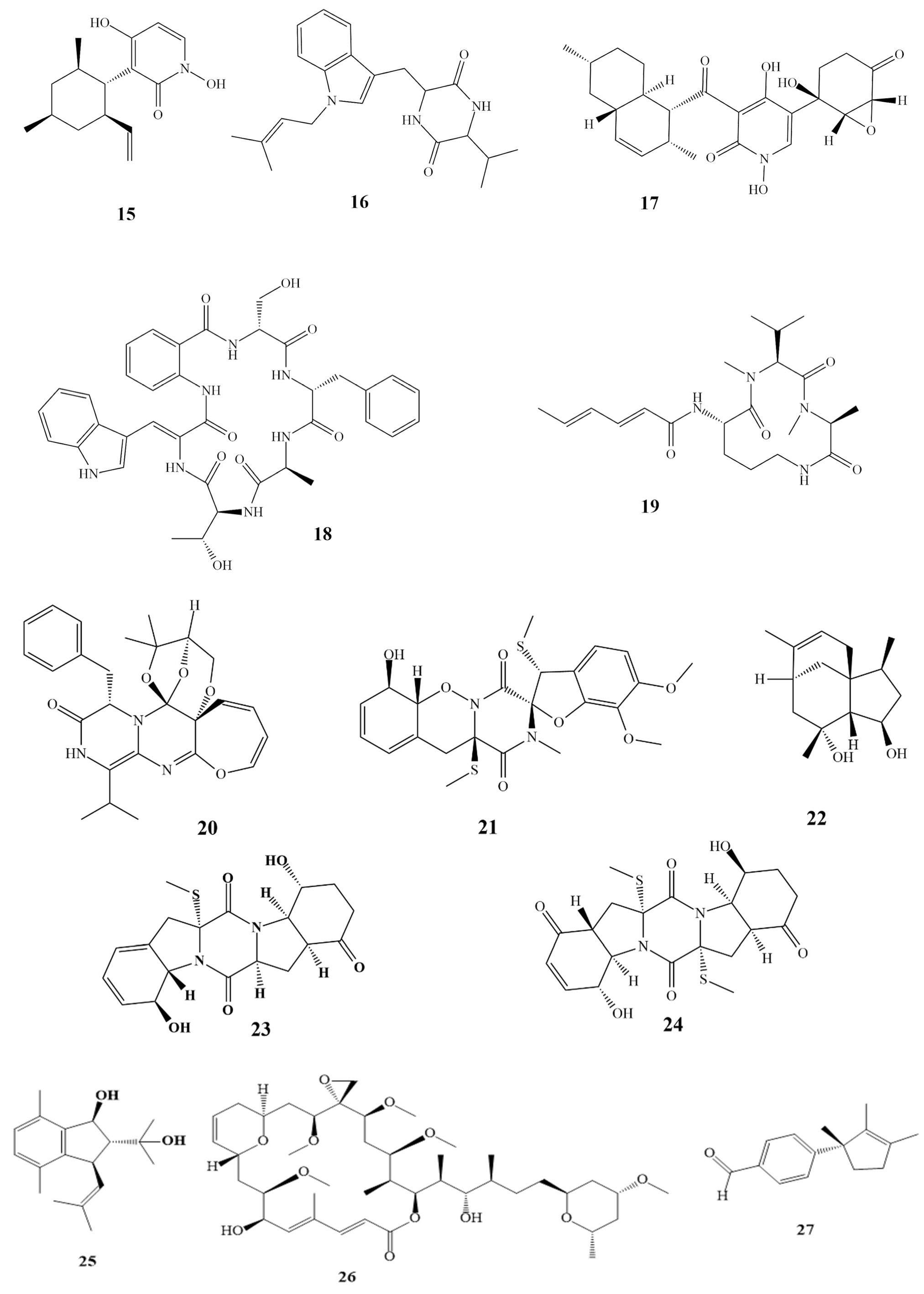
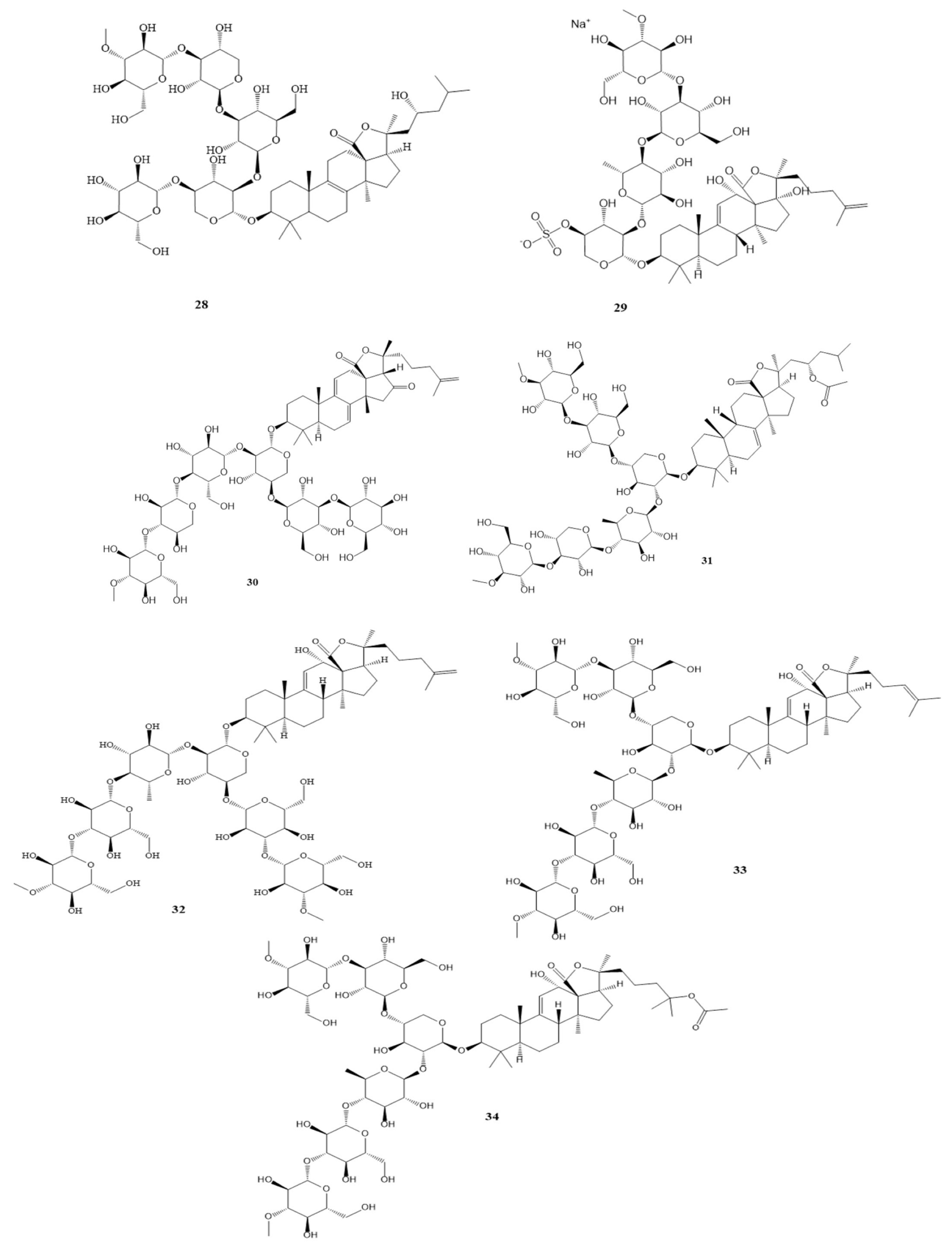
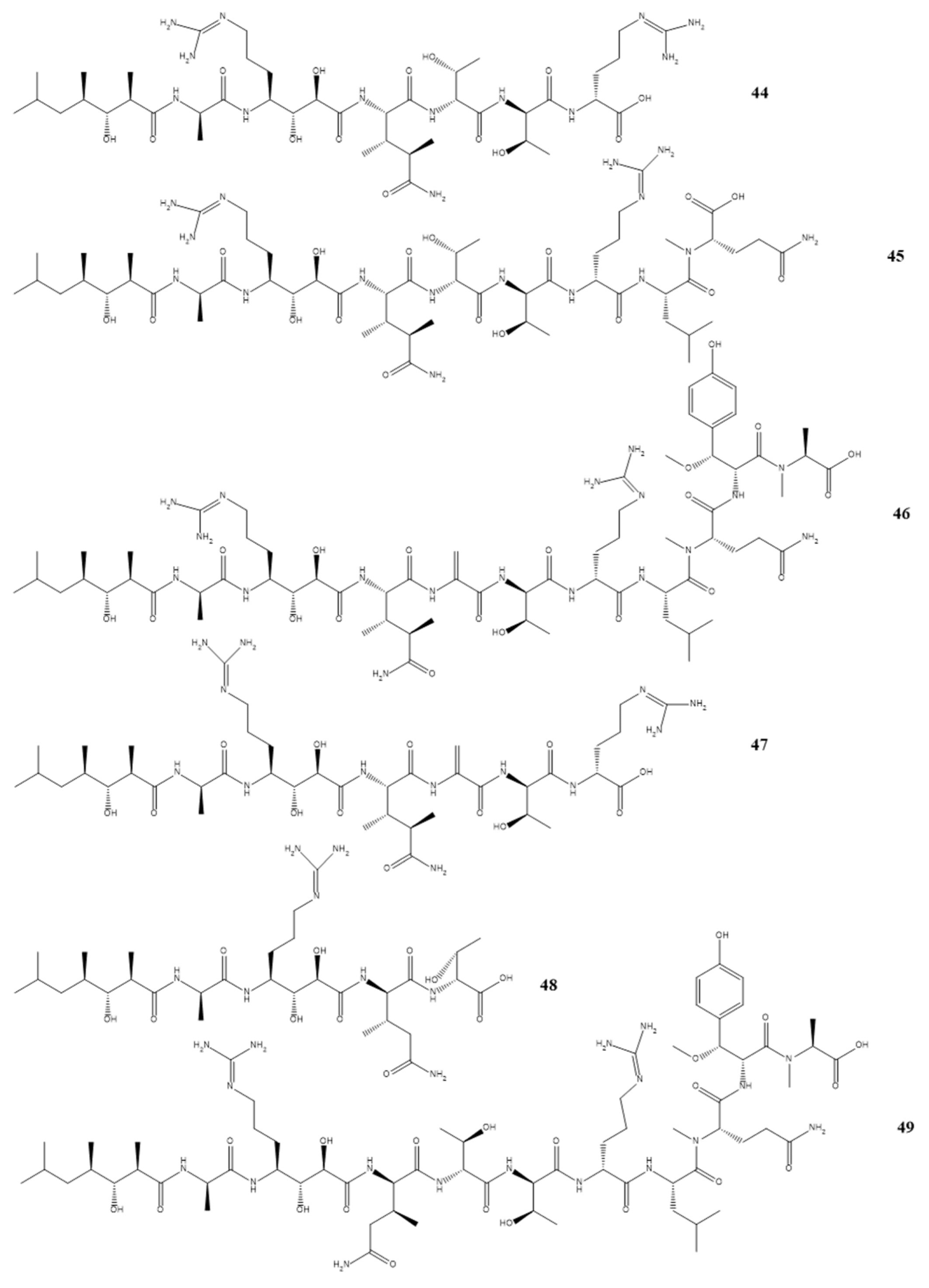
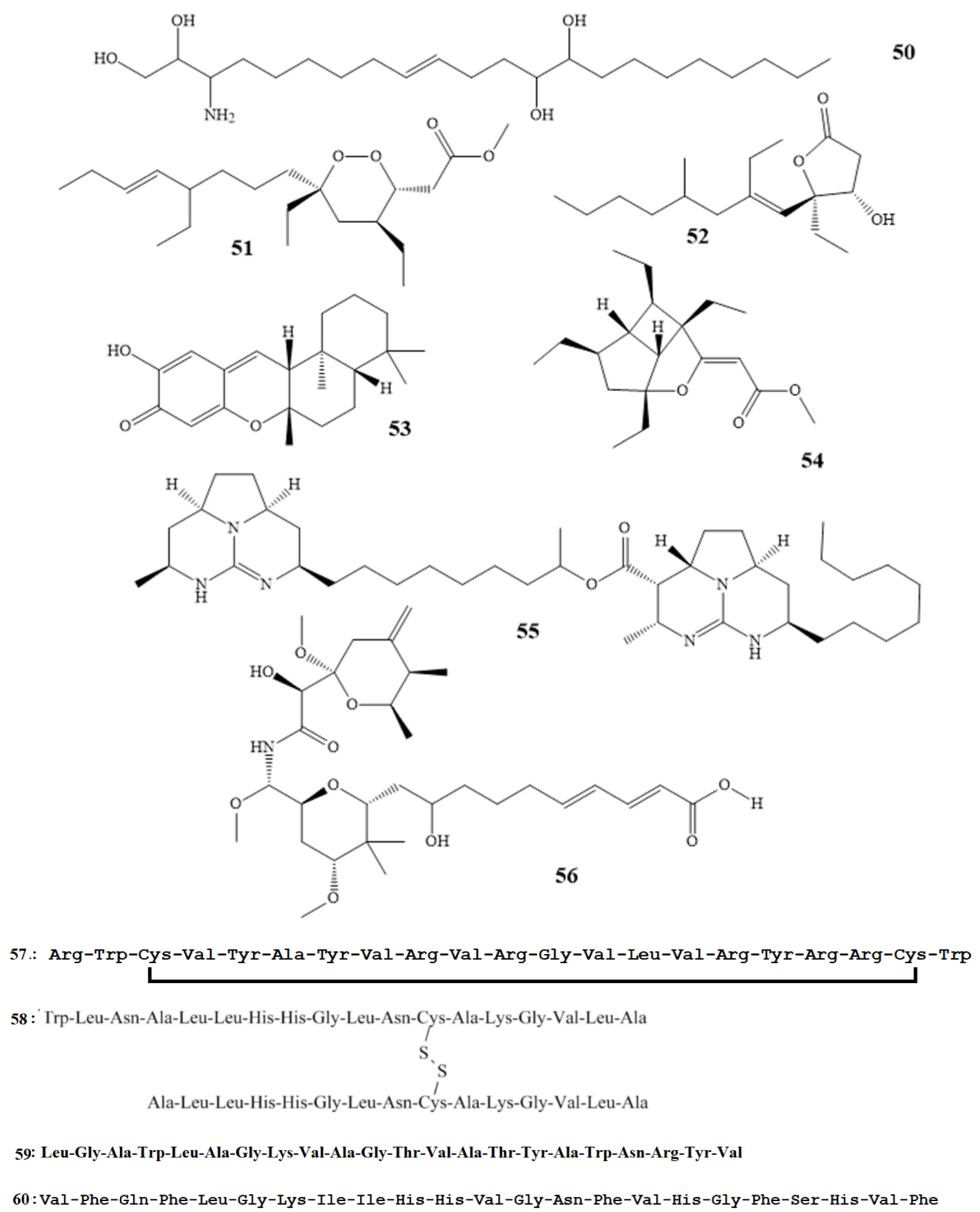
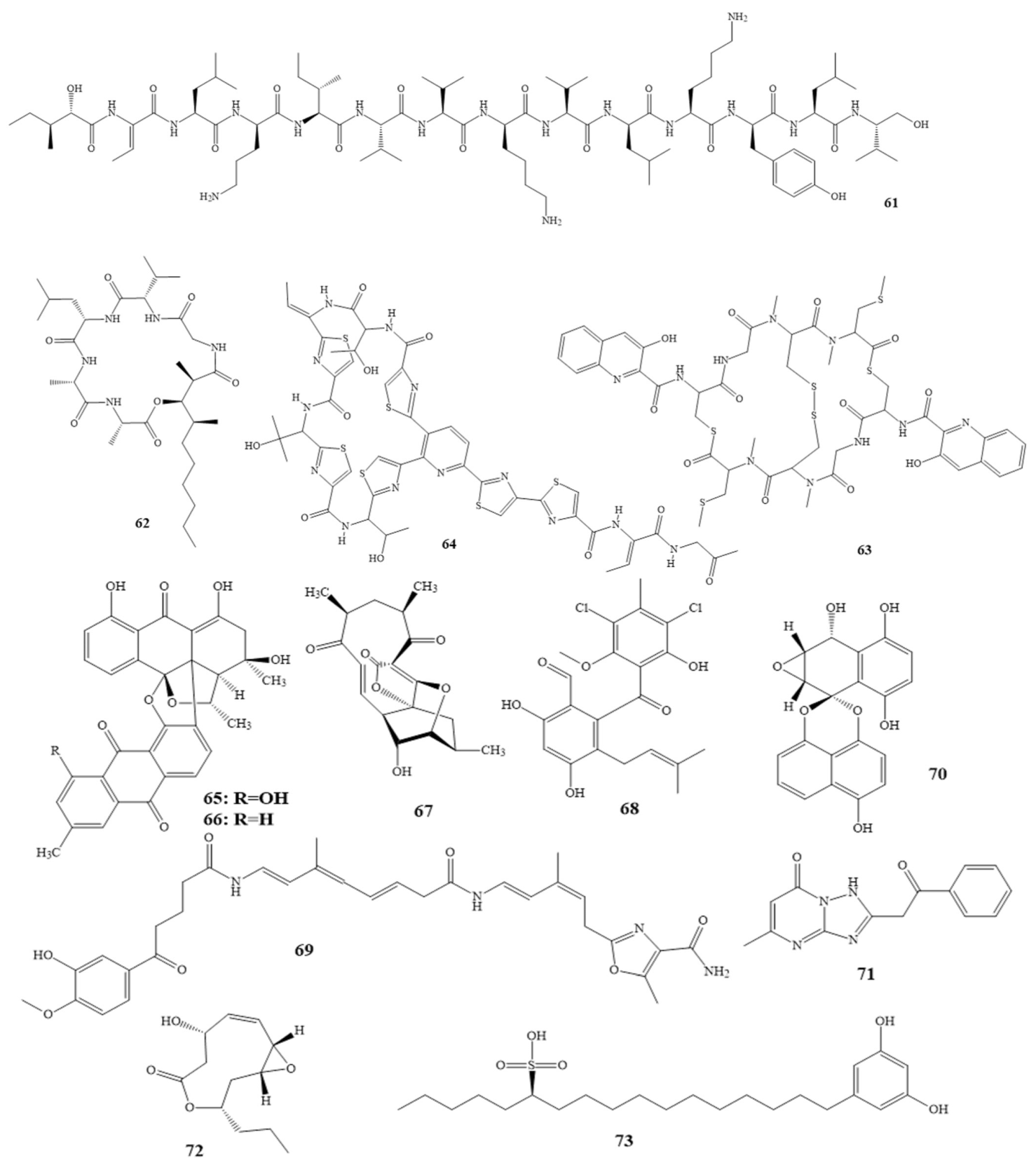
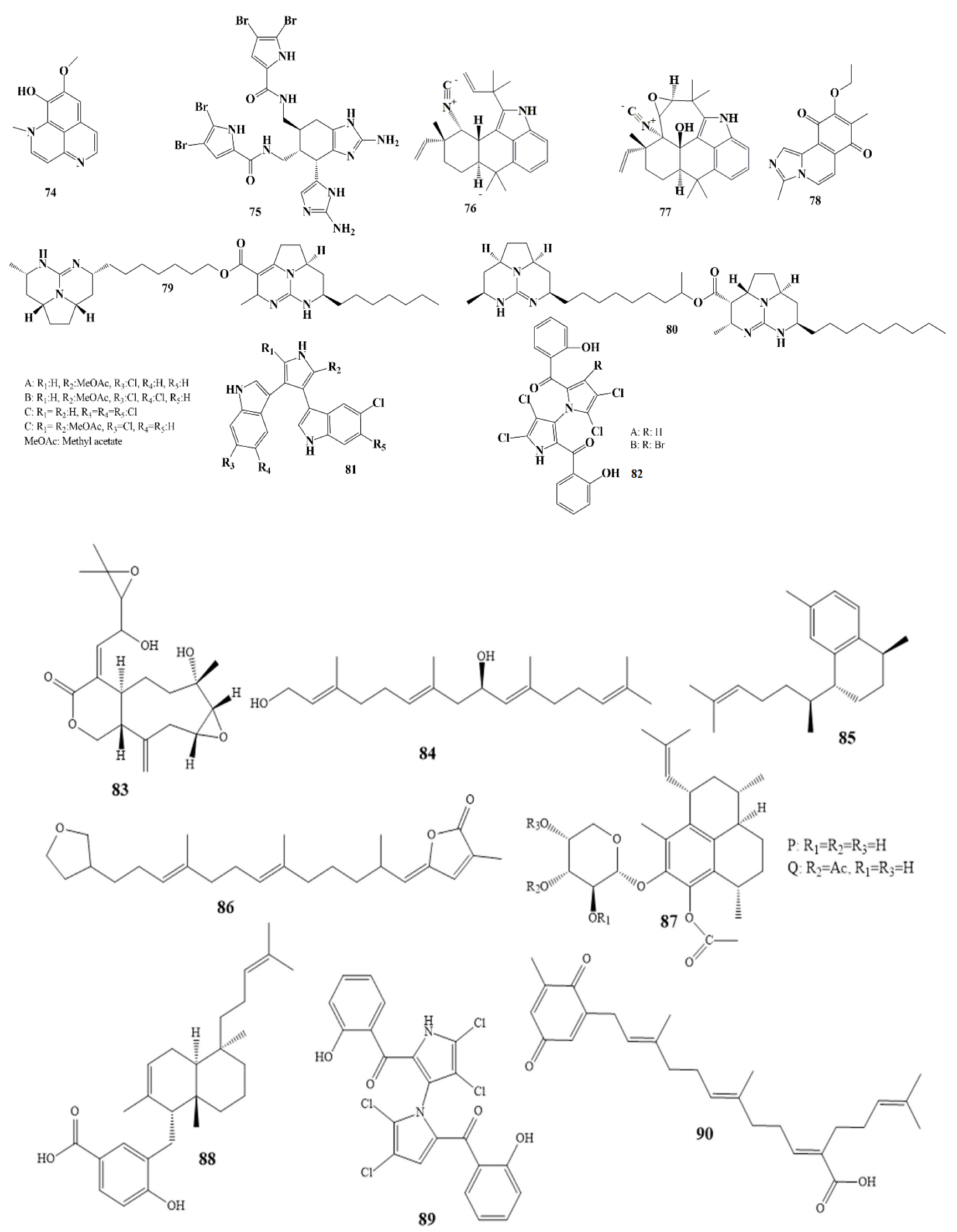
Compounds 7–14 as shown in Scheme 1
2. Antifungal Compounds Isolated from Marine Fungi
3. Antifungal Compounds Isolated from Marine Algae
4. Antifungal Compounds Isolated from Sea Cucumbers
5. Antifungal Compounds Isolated from Sea Sponges
|
Isolated Compound |
Marine Sources |
Ref. |
Conc. of Inhibition |
|---|---|---|---|
|
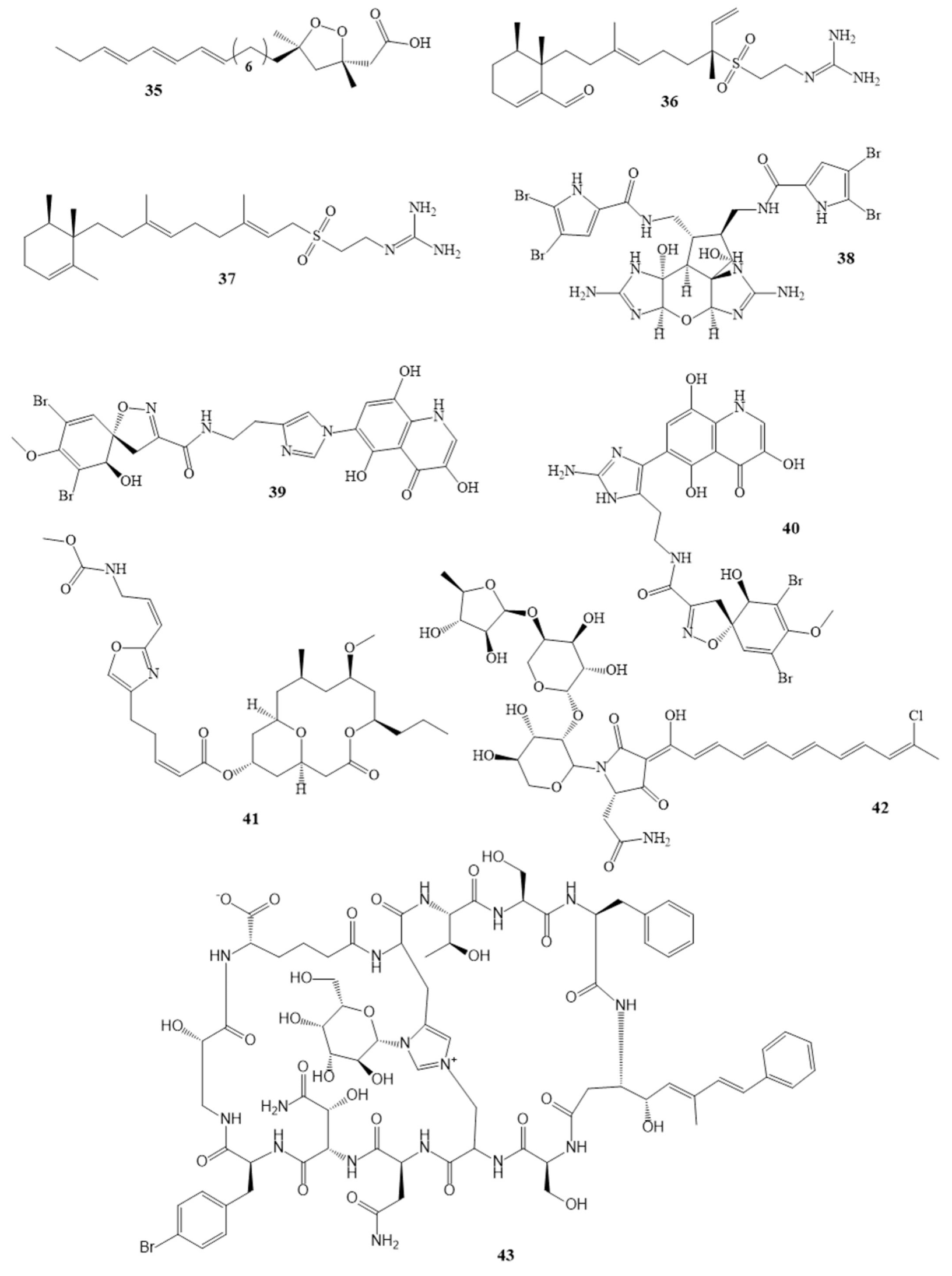
Ceratinadins A (39) Ceratinadins B (40) |
Pseudoceratina sp. |
MIC 2 µg/mL 4 µg/mL |
|
|
Neopeltolide (41) |
Okinawan sponge |
MIC 0.62 µg/mL |
|
|
Theonellamide G (43) |
Theonella swinhoei in the red sea |
[43] |
IC50 4.49 µM |
|
Aurantoside K (42) * |
Melophlus sp. |
[44] |
MIC 31.25 μg/mL |
|
Callipeltins peptides F (44) Callipeltins peptides G (45) Callipeltins peptides H (46) Callipeltins peptides I (47) Callipeltins peptides J (48) Callipeltins peptides K (49) |
Latrunculia sp. sponge usually found in Vanuatu islands and South Pacific |
MIC 100 µM |
This entry is adapted from the peer-reviewed paper 10.3390/toxins15020093
References
- Williams, P.G. Panning for chemical gold: Marine bacteria as a source of new therapeutics. Trends Biotechnol. 2009, 27, 45–52.
- Gulder, T.A.; Moore, B.S. Chasing the treasures of the sea—Bacterial marine natural products. Curr. Opin. Microbiol. 2009, 12, 252–260.
- Joseph, A. Oceans: Abode of Nutraceuticals, Pharmaceuticals, and Biotoxins. In Investigating Seafloors and Oceans; Joseph, A., Ed.; Candice Janco: Goa, India, 2016; pp. 493–554. ISBN 9780128093573.
- Tareq, F.S.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, New Glycolipids from a Marine-Derived Bacterium Bacillus licheniformis 09IDYM23. Lipids 2015, 50, 513–519.
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.; Rai, D.K. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272.
- El-Gendy, M.M.; El-Bondkly, A.M. Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya48. J. Ind. Microbiol. Biotechnol. 2010, 37, 831–841.
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538.
- Ambavane, V.; Tokdar, P.; Parab, R.; Sreekumar, E.S.; Mahajan, G.B.; Mishra, P.D.; Ranadive, P. Caerulomycin A—An antifungal compound isolated from marine actinomycetes. Adv. Microbiol. 2014, 4, 567–578.
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; Hamed, A.N.E.S.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017, 126, 631–651.
- Shin, H.J. Natural products from marine fungi. Mar. Drugs 2020, 18, 230.
- Kumar, V.; Sarma, V.V.; Thambugala, K.M.; Huang, J.J.; Li, X.Y.; Hao, G.F. Ecology and evolution of marine fungi with their adaptation to climate change. Front. Microbiol. 2021, 12, 719000.
- Wu, B.; Oesker, V.; Wiese, J.; Schmaljohann, R.; Imhoff, J.F. Two new antibiotic pyridones produced by a marine fungus, Trichoderma sp. strain MF106. Mar. Drugs 2014, 12, 1208–1219.
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19.
- Borthwick, A.D. 2, 5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716.
- Hu, J.; Li, Z.; Gao, J.; He, H.; Dai, H.; Xia, X.; Liu, C.; Zhang, L.; Song, F. New diketopiperazines from a marine-derived fungus strain Aspergillus versicolor MF180151. Mar. Drugs 2019, 17, 262.
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 2015, 13, 3479–3513.
- Haga, A.; Tamoto, H.; Ishino, M.; Kimura, E.; Sugita, T.; Kinoshita, K.; Koyama, K. Pyridone alkaloids from a marine-derived fungus, Stagonosporopsis cucurbitacearum, and their activities against azole-resistant Candida albicans. J. Nat. Prod. 2013, 76, 750–754.
- Sun, C.; Zhang, Z.; Ren, Z.; Yu, L.; Zhou, H.; Han, Y.; Shah, M.; Che, Q.; Zhang, G.; Li, D.; et al. Antibacterial cyclic tripeptides from Antarctica-sponge-derived fungus Aspergillus insulicola HDN151418. Mar. Drugs 2020, 18, 532.
- Liu, J.; Gu, B.; Yang, L.; Yang, F.; Lin, H. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018, 6, 226.
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76.
- Liu, Y.; Mándi, A.; Li, X.M.; Meng, L.H.; Kurtán, T.; Wang, B.G. Peniciadametizine A, a dithiodiketopiperazine with a unique spiro oxazine] skeleton, and a related analogue, Peniciadametizine B, from the marine sponge-derived fungus Penicillium adametzioides. Mar. Drugs 2015, 13, 3640–3652.
- Meng, L.H.; Li, X.M.; Liu, Y.; Wang, B.G. Penicibilaenes A and B, sesquiterpenes with a tricyclo dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055.
- Meng, L.H.; Zhang, P.; Li, X.M.; Wang, B.G. Penicibrocazines A–E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287.
- Mehner, T.; Krienitz, L. Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 103–113.
- Zerrifi, S.E.A.; El Khalloufi, F.; Oudra, B.; Vasconcelos, V. Seaweed bioactive compounds against pathogens and microalgae: Potential uses on pharmacology and harmful algae bloom control. Mar. Drugs 2018, 16, 55.
- Alarif, W.M.; Al-Lihaibi, S.S.; Ayyad, S.E.N.; Abdel-Rhman, M.H.; Badria, F.A. Laurene-type sesquiterpenes from the Red Sea red alga Laurencia obtusa as potential antitumor–antimicrobial agents. Eur. J. Med. Chem. 2012, 55, 462–466.
- Raeesossadati, M.J.; Ahmadzadeh, H.; McHenry, M.P.; Moheimani, N.R. CO2 bioremediation by microalgae in photobioreactors: Impacts of biomass and CO2 concentrations, light, and temperature. Algal Res. 2014, 6, 78–85.
- De Almeida, C.L.F.; Falcão, H.D.S.; Lima, G.R.D.M.; Montenegro, C.D.A.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.D.F.V.D.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573.
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55–57.
- Wang, X.H.; Zou, Z.R.; Yi, Y.H.; Han, H.; Li, L.; Pan, M.X. Variegatusides: New non-sulphated triterpene glycosides from the sea cucumber Stichopus variegates semper. Mar. Drugs 2014, 12, 2004–2018.
- Bahrami, Y.; Franco, C.M. Acetylated triterpene glycosides and their biological activity from holothuroidea reported in the past six decades. Mar. Drugs 2016, 14, 147.
- Hua, H.A.N.; Ling, L.I.; Yi, Y.H.; Wang, X.H.; Pan, M.X. Triterpene glycosides from sea cucumber Holothuria scabra with cytotoxic activity. Chin. Herb. Med. 2012, 4, 183–188.
- Wang, Z.N.; Yuan, X. Concurrent effects of hot streak and gas species concentration on aerothermal characteristics in a turbine stage. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2012; Volume 44748, pp. 1431–1441.
- Elbandy, M.; Rho, J.R.; Afifi, R. Analysis of saponins as bioactive zoochemicals from the marine functional food sea cucumber Bohadschia cousteaui. Eur. Food Res. Technol. 2014, 238, 937–955.
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805.
- Jamison, M.T.; Dalisay, D.S.; Molinski, T.F. Peroxide natural products from plakortis zyggompha and the sponge association plakortis halichondrioides–xestospongia deweerdtae: Antifungal activity against Cryptococcus gattii. J. Nat. Prod. 2016, 79, 555–563.
- Stout, E.P.; Yu, L.C.; Molinski, T.F. Antifungal diterpene alkaloids from the Caribbean sponge Agelas citrina: Unified configurational assignments of agelasidines and agelasines. Eur. J. Org. Chem. 2012, 2012, 5131–5135.
- Zhou, X.; Hartman, S.V.; Born, E.J.; Smits, J.P.; Holstein, S.A.; Wiemer, D.F. Triazole-based inhibitors of geranylgeranyltransferase II. Bioorganic Med. Chem. Lett. 2013, 23, 764–766.
- Gotsbacher, M.P.; Karuso, P. New antimicrobial bromotyrosine analogues from the sponge Pseudoceratina purpurea and its predator Tylodina corticalis. Mar. Drugs 2015, 13, 1389–1409.
- Olatunji, O.J. Bromotyrosines from the sponges Acanthodendrilla sp. and Pseudoceratina cf. Ph.D. Thesis, Prince of Songkla University, Hat Yai, Thailand, 2014.
- Fuwa, H. Contemporary strategies for the synthesis of tetrahydropyran derivatives: Application to total synthesis of neopeltolide, a marine macrolide natural product. Mar. Drugs 2016, 14, 65.
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129.
- Youssef, D.T.; Shaala, L.A.; Mohamed, G.A.; Badr, J.M.; Bamanie, F.H.; Ibrahim, S.R. Theonellamide G, a potent antifungal and cytotoxic bicyclic glycopeptide from the Red Sea marine sponge Theonella swinhoei. Mar. Drugs 2014, 12, 1911–1923.
- Kumar, R.; Subramani, R.; Feussner, K.D.; Aalbersberg, W. Aurantoside K, a new antifungal tetramic acid glycoside from a Fijian marine sponge of the genus Melophlus. Mar. Drugs 2012, 10, 200–208.
- Kikuchi, M.; Nosaka, K.; Akaji, K.; Konno, H. Solid phase total synthesis of callipeltin isolated from marine sponge Latrunculia sp. Tetrahedron Lett. 2011, 52, 3872–3875.
- Stierhof, M.; Hansen, K.Ø.; Sharma, M.; Feussner, K.; Subko, K.; Díaz-Rullo, F.F.; Isaksson, J.; Pérez-Victoria, I.; Clarke, D.; Hansen, E.; et al. New cytotoxic callipeltins from the Solomon Island marine sponge Asteropus sp. Tetrahedron 2016, 72, 6929–6934.
- El-Amraoui, B.; Biard, J.F.; Fassouane, A. Haliscosamine: A new antifungal sphingosine derivative from the Moroccan marine sponge Haliclona viscosa. Springerplus 2013, 2, 252.
- Xu, T.; Feng, Q.; Jacob, M.R.; Avula, B.; Mask, M.M.; Baerson, S.R.; Tripathi, S.K.; Mohammed, R.; Hamann, M.T.; Khan, I.A.; et al. The marine sponge-derived polyketide endoperoxide plakortide F acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob. Agents Chemother. 2011, 55, 1611–1621.
- Liu, X.F.; Shen, Y.; Yang, F.; Hamann, M.T.; Jiao, W.H.; Zhang, H.J.; Chen, W.-S.; Lin, H.W. Simplexolides A–E and plakorfuran A, six butyrate derived polyketides from the marine sponge Plakortis simplex. Tetrahedron 2012, 68, 4635–4640.
- Tripathi, S.K.; Feng, Q.; Liu, L.; Levin, D.E.; Roy, K.K.; Doerksen, R.J.; Baerson, S.R.; Shi, X.; Pan, X.; Xu, W.-H.; et al. Puupehenone, a marine-sponge-derived sesquiterpene quinone, potentiates the antifungal drug caspofungin by disrupting Hsp90 activity and the cell wall integrity pathway. Msphere 2020, 5, e00818-19.
- Piao, S.J.; Song, Y.L.; Jiao, W.H.; Yang, F.; Liu, X.F.; Chen, W.S.; Han, B.-N.; Lin, H.W. Hippolachnin A, a new antifungal polyketide from the South China sea sponge Hippospongia lachne. Org. Lett. 2013, 15, 3526–3529.
- Arevabini, C.; Crivelenti, Y.D.; de Abreu, M.H.; Bitencourt, T.A.; Santos, M.F.; Berlinck, R.G.; Marins, M. Antifungal activity of metabolites from the marine sponges Amphimedon sp. and Monanchora arbuscula against Aspergillus flavus strains isolated from peanuts (Arachis hypogaea). Nat. Prod. Commun. 2014, 9, 33–36.
- Mosey, R.A.; Floreancig, P.E. Isolation, biological activity, synthesis, and medicinal chemistry of the pederin/mycalamide family of natural products. Nat. Prod. Rep. 2012, 29, 980–995.
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41.
