Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cenobamate (CNB), ([(R)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl], is a novel tetrazole alkyl carbamate derivative. In November 2019, the Food and Drug Administration approved Xcopri®, marketed by SK Life Science Inc., (Paramus, NJ, USA) for adult focal seizures. The European Medicines Agency approved Ontozry® by Arvelle Therapeutics Netherlands B.V.(Amsterdam, The Neatherlands) in March 2021. Cenobamate is a medication that could potentially change the perspectives regarding the management and prognosis of refractory epilepsy.
- cenobamate
- YKP3089
- Xcopri
- Ontozry
- epilepsy
1. Introduction
Epilepsy affects more than seventy million individuals worldwide, corresponding to an age-standardized prevalence of 621.5 per 100,000 people [1]. Approximately 3 million adults and almost 500,000 children in the United States have epilepsy [2]. Increased life expectancies and more people surviving events that can lead to epilepsy are expected to raise the number of people with epilepsy [3]. The estimated annual costs in the United States of acute seizure care are around USD 12.5 billion [4]. In this context, the burden of drug-resistant epilepsy (DRE) is believed to be significantly higher due to the number of antiseizure medications (ASMs) used concomitantly and the possible high incidence of adverse events [5][6].
Seizure freedom is a primary goal in the treatment of epilepsy. Only half of the individuals with epilepsy will become seizure-free with their first ASM [7]. Also, more than one in every three patients with epilepsy will have uncontrolled seizures despite adequate management and anticonvulsant therapy [8]. In this context, failure to achieve sustained seizure freedom with the rational use of two anti-seizure drugs administered alone or in combination defines drug-resistant epilepsy [9].
Uncontrolled epilepsy, compared to epilepsy in general, is associated with ten-to-fifteen-fold more frequent mortality secondary to traumatic injury, drowning, suicide, and sudden unexpected death from epilepsy (SUDEP) [10]. Also, some types of childhood epilepsies are related to neuronal damage leading to epileptic encephalopathy, resulting in lifelong disabilities [11]. In addition, low employment rates and lower high school graduation rates can hinder individuals with epilepsy from reaching their maximum potential [12]. Therefore, poor control of seizures can lead to a higher risk of experiencing physical and psychological disorders, causing worse healthcare outcomes, increased healthcare needs, and decreased quality of life [13].
Many new ASMs have been discovered during the last three decades, with more than twenty new ASMs approved [14]. In this context, these new anticonvulsants have improved the spectrum of side effects, increased routes of administration, and reduced the severity of epilepsy, leading to better compliance and treatment adherence [15][16]. But, there were no significant changes in the proportion of individuals affected by DRE. Interestingly, the prevalence of DRE in the 1980s was sixty-three percent, and in 2014 this number was sixty-four percent [17].
2. Historical Aspects of Cenobamate
Epilepsy is a neurological disorder characterized by recurrent and unprovoked seizures [18]. It is believed that excessive excitability in neural tissues can contribute to the abnormal electrical activities leading to epilepsy [19]. ASMs acting on voltage-gated sodium channels have been utilized for the pharmacologic management of epilepsy because these channels are essential for generating and conducting action potentials [20]. Also, several point mutations in voltage-gated sodium channels, which exhibit increased persistent sodium currents, have been identified in patients with epilepsy [21]. Some ASMs, such as phenytoin and lamotrigine, are known to affect persistent sodium currents [22].
In 1951, during rat studies to develop a new anxiolytic drug, meprobamate was observed to have antiseizure activity [23]. Ten years later, Frank Berger at Wallace Laboratories noted the remarkable efficacy of felbamate in controlling abnormal electrical activity in animal models of epilepsy (Figure 1) [24]. In 2008, Johnson & Johnson submitted a new application for carisbamate, which was approved by the U.S. Food and Drug Administration [25]. But, two years later, carisbamate was removed from the market due to insignificant superiority over a placebo in a randomized controlled trial [26].
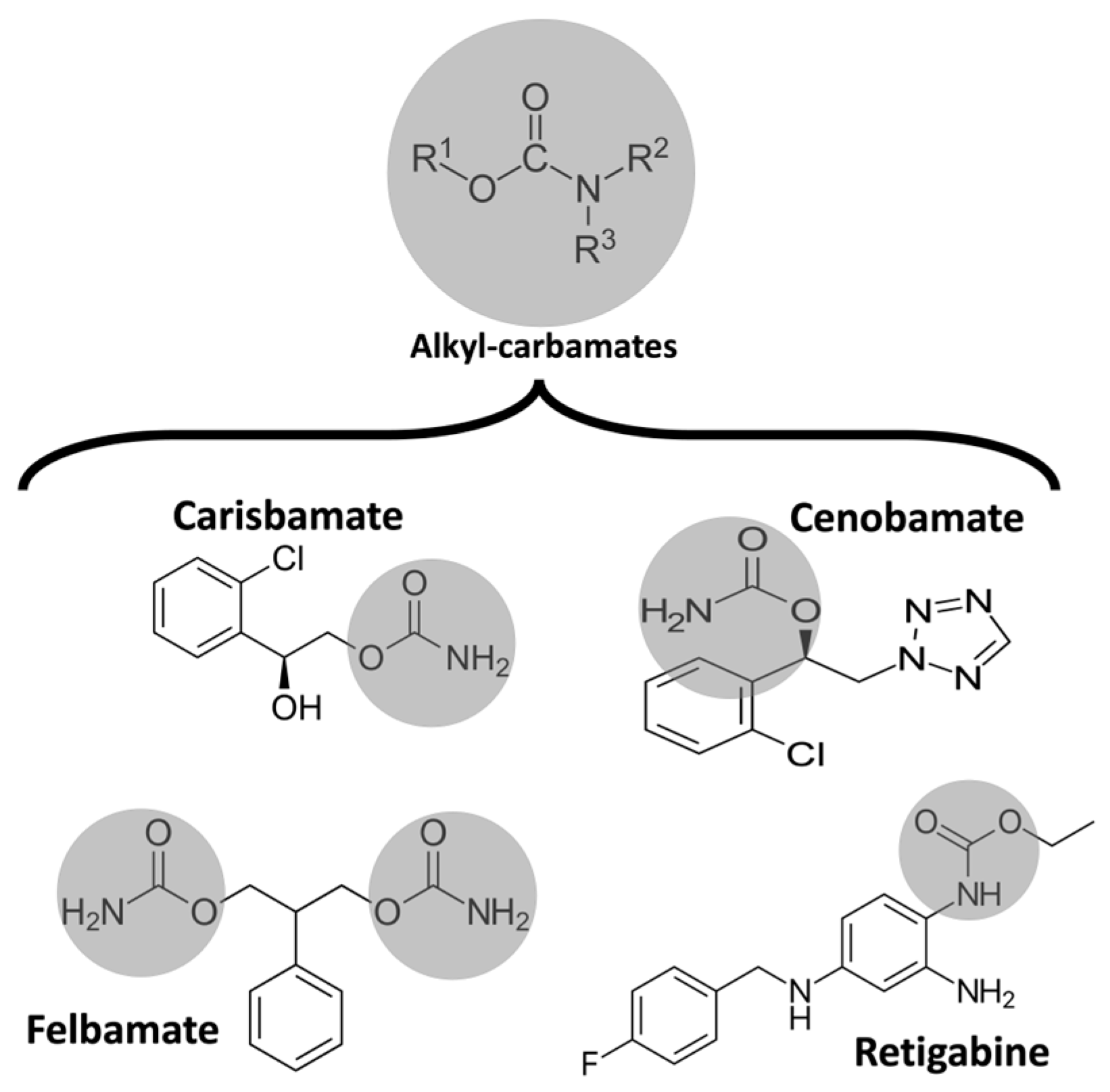
Figure 1. Chemical structure of some alkyl-carbamates with antiseizure activity. Carisbamate, cenobamate, felbamate, and retigabine (ezogabine). Note that felbamate is a dicarbamate. The other drugs are monocarbamates.
Cenobamate (CNB), ([(R)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl], is a novel tetrazole alkyl carbamate derivative [27]. It showed antiseizure activity in the maximal electroshock test and prevented seizures induced by chemical convulsants such as pentylenetetrazol and picrotoxin [28]. Also, CNB was reported to be effective in two models of focal seizure, the hippocampal kindled rat and the mouse 6 Hz psychomotor seizure models [29]. Moreover, CNB has been reported to effectively and dose-dependently reduce the number and cumulative duration of spike-and-wave discharges characteristic of absence seizures in genetic absence epilepsy rats in the Strasbourg (GAERS) model [30].
CNB differs from other broad-spectrum ASMs because it has a sustained efficacy and potency in the 6 Hz test regardless of the stimulus intensity. Löscher et al., believed this significant efficacy predicted good outcomes in clinical trials [31]. In this way, the 6 Hz test should be studied as a therapy-resistant seizure model, and other ASMs should be assessed for a complete understanding of this model in DRE. Therefore, the significant efficacy of CNB in different models suggests that this drug may have a broad spectrum of activity [32].
The photosensitivity model has been established as a “proof-of-concept” study to achieve a reliable prediction of potential efficacy and chronic-use dosing of the early period of clinical trials with new ASMs. In this model, people with epilepsy are randomly distributed to take the tested drug or placebo as an adjunct single dose. An electroencephalogram (EEG) is used to observe abnormalities in the photoparoxysmal response [33]. Trenite et al., performed a single-blind non-randomized study with the photoparoxismal-EEG response (PPR) model in seven individuals with photosensitive epilepsy after oral doses of CNB of 100, 250, and 400 mg or placebo. A complete suppression of PPR response in photosensitive individuals at 250 and 400 mg single doses of CNB was observed. The subjects taking the CNB 100 mg dose had only partially suppressed PPR [34]. Thus, these results provide evidence of CNB’s potential efficacy in managing seizures in patients with epilepsy and support the clinical trials with this medication.
New methods to determine the plasma levels of CNB were developed to assess the pharmacokinetics of this drug in clinical trials. The first high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was designed [35]. Carisbamate was used as the internal standard, and the preparation of plasma samples required the precipitation of proteins by acetonitrile. The calibration curve of this method was linear over a concentration range of 10–5000 ng/mL [36]. An achiral LC-MS/MS method was validated in heparinized plasma samples for pharmacokinetic studies. Phenacetin was used as an internal standard, and plasma samples with precipitation of proteins were analyzed. A third method of LC-MS/MS was developed for pharmacokinetic studies of CNB in plasma after administering a single-capsule formulation of 400 mg. The calibration curve range was 0.080–40.0 mg/L [37]. The fourth method to quantify CNB in human plasma samples was developed using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. The calibration curve range was 0.050–20.0 mg/L [38].
The first CNB trials revealed that more than twenty percent of people with epilepsy became seizure-free. Interestingly, this seizure freedom has never been reported in a placebo-controlled, double-blind trial of anticonvulsive drugs [39]. In this context, the FDA decision regarding CNB approval for marketing was remarkable. The advisory board recommended that additional randomized controlled phase 3 trials to investigate the efficacy of CNB were not necessary because of the impressive efficacy data in the phase 2 trials. Also, they replied that open-label safety data should be performed because of some rare cases of drug rash with eosinophilia and systemic symptoms (DRESS) in early trials [40][41].
The development of new routes of drug administration is important for the improvement of adherence. The administration of medications via enteral feeding tubes may be necessary for patients who cannot swallow safely, such as individuals with dysphagia due to cognitive impairment or physical disability. Ferrari et al., studied the recovery of CNB after the administration of a suspension prepared from filmcoated tablets via ex vivo nasogastric and gastrostomy feeding tubes. The authors observed that the mean percentage of recovery from CNB was within the predetermined acceptable range (90.0–110.0%), which can suggest no adhesion or adsorption of CNB to enteral feeding tubes [42]. Thus, enteral feeding tubes may be suitable for the administration of CNB.
3. Pharmacology and Mechanism of Action
CNB’s mechanism of action has yet to be completely understood. Interestingly, CNB was discovered purely by phenotype-based screening, and its presumed dual mechanism of action was only described years after the first studies [43]. CNB can reduce repetitive neuronal firing by inhibiting voltage-gated sodium currents. It may enhance the fast and slow inactivation of sodium channels and potently inhibit the non-inactivating persistent component of the sodium channel current, which has already been observed with other ASMs [44]. Noteworthily, CNB had little effect on the peak component of transient sodium currents induced by brief depolarizing step pulses. But, CNB strongly inhibited the noninactivating persistent component of sodium currents [45]. Therefore, CNB may modify excitability in principal neurons without compromising inhibitory interneurons [46]. Also, CNB was revealed to be a positive allosteric modulator of the γ-aminobutyric acid (GABA) ion channel. This effect was similar for all tested GABAA receptors containing six different alpha subunits (α1β2γ2 or α2-6β3γ2).
Nakamura et al., studied the effects of CNB in rat hippocampal CA3 neurons. They observed that CNB had little effect on the peak component of transient sodium current induced by brief depolarizing step pulses. Still, CNB potently inhibited the non-inactivating persistent component of sodium currents. Also, it inhibited the sodium currents evoked by slow voltage-ramp stimuli [45]. Noteworthily, the effect of CNB in sodium current in hippocampal rat neurons was concentration-dependent [40].
Sharma et al., assessed the effects of CNB on GABAergic neurotransmission, specifically its effects on GABAA receptors mediating inhibitory postsynaptic currents and tonic conductance in rodent hippocampal neurons. The authors found that CNB is a positive allosteric modulator of high-affinity GABAA receptors, activated by GABA at a site independent of the benzodiazepine binding site, and efficiently enhances tonic conductance inhibition in hippocampal neurons [47]. CNB may resemble barbiturate action because of increased tonic and phasic inhibition through GABAA receptor activation [48]. It is worth mentioning that these mechanisms were already observed in animal studies with neurosteroids [49]. Also, the effect on both phases of GABAA receptor activation could partially explain the efficacy of CNB in managing status epilepticus [50].
The CNB terminal half-life of 50 to 60 h allows this drug to be taken once a day. Noteworthily, this terminal half-life increases with increasing doses of CNB from 30 h (CNB 10 mg) to 76 h (CNB 750 mg). The area under the plasma concentration versus time curve (AUC) increases more than proportionally after the administration of single doses of CNB ranging from 5 to 750 mg. However, after multiple doses of CNB at the steady state, AUC increases linearly with increasing doses within the 50–500 mg/day dose range. Plasma CNB concentrations are steady after approximately two weeks of once-daily dosing. The tablets should be swallowed whole and not crushed or chewed.
CNB pharmacokinetics have been reported to be consistent regarding gender, race, and age. Patients with mild-to-moderate (Clcr 30 to 90 mL/min) and severe (Clcr 30 mL/min) renal impairment and those with mild-to-moderate hepatic impairment should be treated with caution and reduced dose. There are no data regarding CNB’s pharmacokinetics in individuals with end-stage renal disease (Clcr < 15 mL/min) undergoing hemodialysis and those with severe hepatic impairment.
Vernillet et al., studied the mass balance and the metabolic profiling of CNB in humans. Eight CNB metabolites (M1, M2a, M2b, M3, M5, M6, M7, and M11) were identified across plasma, urine, and feces. CNB was the main plasma radioactive component, and M1 was the only metabolite detected in plasma (>98% and <2% total radioactivity AUC, respectively). All detected metabolites were found in urine; unchanged CNB accounted for approximately six percent. CNB metabolites appeared to be formed slowly [37]. Greene et al., assessed the effect of CNB on the single-dose pharmacokinetics of multiple cytochrome P450 probes in healthy subjects. They observed that CNB induces CYP2B6 activity, exhibits a dose-dependent induction of CYP3A4/5 activity, inhibits CYP2C19 activity, and has a negligible effect on CYP2C9 activity [51].
Researchers calculated the chemical and pharmacological properties of CNB using the SwissADME tool (Figure 2). These properties can help identify compounds suitable for oral use [52]. All the parameters analyzed were within the normal range, except for an insaturation slightly higher than those desired for oral molecules, which can reduce oral bioavailability.
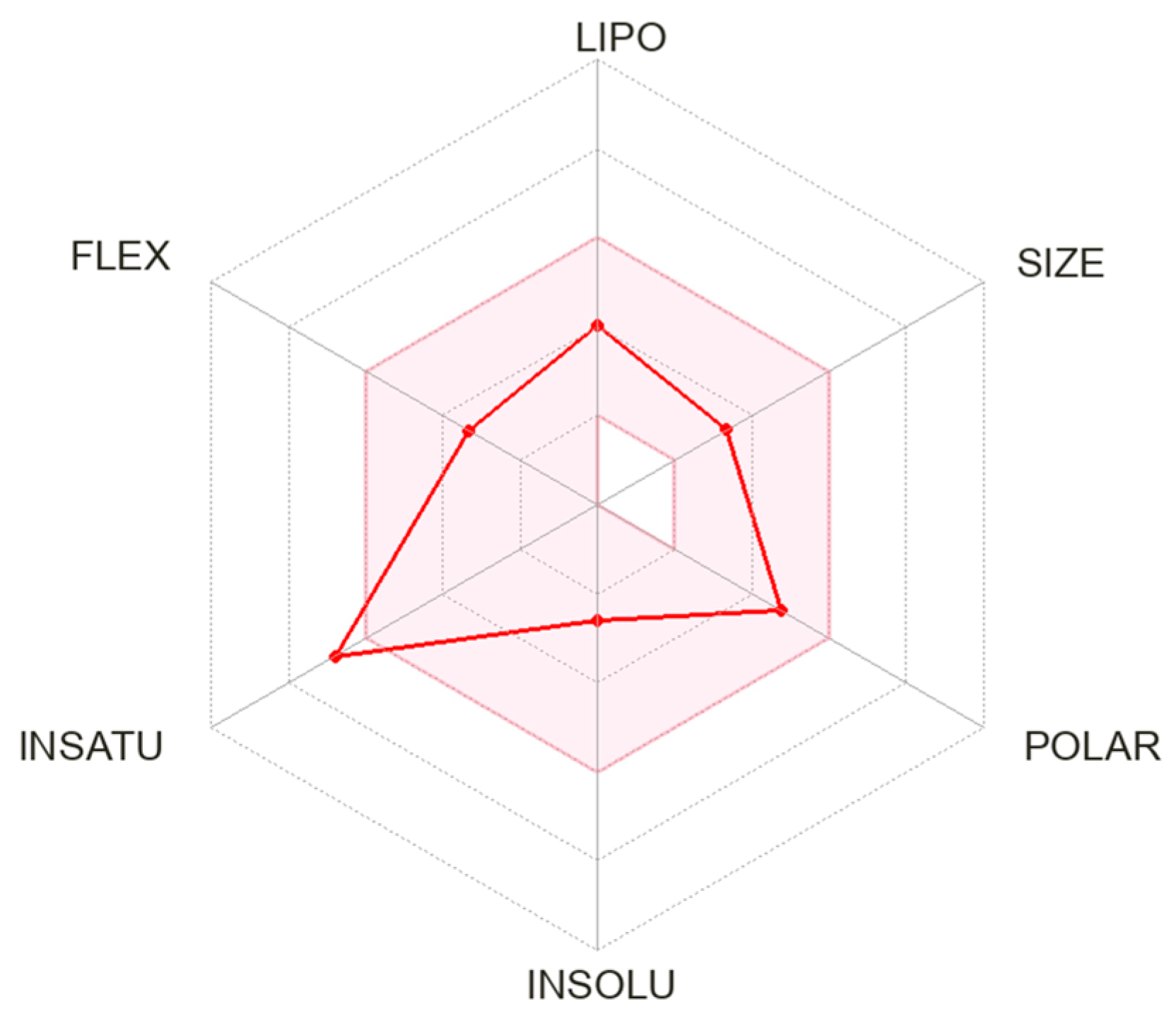
Figure 2. Physicochemical properties of cenobamate. The pink area represents the optimal range for each property. Abbreviation: LIPO: lipophilicity; FLEX: flexibility; INSATU: saturation; INSOLU: solubility.
This entry is adapted from the peer-reviewed paper 10.3390/medicina59081389
References
- GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375.
- Zack, M.M.; Kobau, R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 821–825.
- Beghi, E.; Giussani, G.; Costa, C.; DiFrancesco, J.C.; Dhakar, M.; Leppik, I.; Kwan, P.; Akamatsu, N.; Cretin, B.; O’Dwyer, R.; et al. The epidemiology of epilepsy in older adults: A narrative review by the ILAE Task Force on Epilepsy in the Elderly. Epilepsia 2023, 64, 586–601.
- Begley, C.E.; Durgin, T.L. The direct cost of epilepsy in the United States: A systematic review of estimates. Epilepsia 2015, 56, 1376–1387.
- Guery, D.; Rheims, S. Clinical Management of Drug Resistant Epilepsy: A Review on Current Strategies. Neuropsychiatr. Dis. Treat. 2021, 17, 2229–2242.
- Pong, A.W.; Ross, J.; Tyrlikova, I.; Giermek, A.J.; Kohli, M.P.; Khan, Y.A.; Salgado, R.D.; Klein, P. Epilepsy: Expert opinion on emerging drugs in phase 2/3 clinical trials. Expert Opin. Emerg. Drugs 2022, 27, 75–90.
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286.
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319.
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077.
- Mesraoua, B.; Deleu, D.; Hassan, A.H.; Gayane, M.; Lubna, A.; Ali, M.A.; Tomson, T.; Khalil, B.A.; Cross, J.H.; Asadi-Pooya, A.A. Dramatic outcomes in epilepsy: Depression, suicide, injuries, and mortality. Curr. Med. Res. Opin. 2020, 36, 1473–1480.
- Specchio, N.; Curatolo, P. Developmental and epileptic encephalopathies: What we do and do not know. Brain 2021, 144, 32–43.
- Morrell, M.J. Stigma and epilepsy. Epilepsy Behav. 2002, 3, 21–25.
- Kanner, A.M. Depression and epilepsy: A new perspective on two closely related disorders. Epilepsy Curr. 2006, 6, 141–146.
- Kanner, A.M.; Bicchi, M.M. Antiseizure Medications for Adults with Epilepsy: A Review. JAMA 2022, 327, 1269–1281.
- French, J.A. Cenobamate for focal seizures—A game changer? Nat. Rev. Neurol. 2020, 16, 133–134.
- Perucca, E.; Brodie, M.J.; Kwan, P.; Tomson, T. 30 years of second-generation antiseizure medications: Impact and future perspectives. Lancet Neurol. 2020, 19, 544–556.
- Ungar, A.; Ceccofiglio, A.; Pescini, F.; Mussi, C.; Tava, G.; Rafanelli, M.; Langellotto, A.; Marchionni, N.; Dijk, J.G.; Galizia, G.; et al. Syncope and Epilepsy coexist in ‘possible’ and ‘drug-resistant’ epilepsy (Overlap between Epilepsy and Syncope Study—OESYS). BMC Neurol. 2017, 17, 45.
- Berg, A.T. Febrile seizures and epilepsy: The contributions of epidemiology. Paediatr. Perinat. Epidemiol. 1992, 6, 145–152.
- Das, N.; Dhanawat, M.; Shrivastava, S.K. An overview on antiepileptic drugs. Drug Discov. Ther. 2012, 6, 178–193.
- Catterall, W.A. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 317–338.
- Lossin, C.; Wang, D.W.; Rhodes, T.H.; Vanoye, C.G.; George, A.L. Molecular basis of an inherited epilepsy. Neuron 2002, 34, 877–884.
- Stafstrom, C.E. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007, 7, 15–22.
- Ramchandani, D.; López-Muñoz, F.; Alamo, C. Meprobamate-tranquilizer or anxiolytic? A historical perspective. Psychiatr. Q. 2006, 77, 43–53.
- Ludwig, B.J.; Powell, L.S.; Berger, F.M. Carbamate derivatives related to meprobamate. J. Med. Chem. 1969, 12, 462–472.
- Levy, R.; Ragueneau-Majlessi, I.; Solanki, B.; Zannikos, P.; Yao, C.; Novak, G. Pharmacokinetics, safety, and tolerability of the new antiepileptic carisbamate in the elderly. Epilepsy Res. 2008, 79, 22–30.
- Sperling, M.R.; Greenspan, A.; Cramer, J.A.; Kwan, P.; Kälviäinen, R.; Halford, J.J.; Schmitt, J.; Yuen, E.; Cook, T.; Haas, M.; et al. Carisbamate as adjunctive treatment of partial onset seizures in adults in two randomized, placebo-controlled trials. Epilepsia 2010, 51, 333–343.
- Zaccara, G.; Lattanzi, S.; Leo, A.; Russo, E. Critical Appraisal of Cenobamate as Adjunctive Treatment of Focal Seizures in Adults. Neuropsychiatr. Dis. Treat. 2021, 17, 3447–3457.
- Bialer, M.; Johannessen, S.I.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res. 2013, 103, 2–30.
- Keam, S.J. Cenobamate: First Approval. Drugs 2020, 80, 73–78.
- Barbieri, M.A.; Perucca, E.; Spina, E.; Rota, P.; Franco, V. Cenobamate: A Review of its Pharmacological Properties, Clinical Efficacy and Tolerability Profile in the Treatment of Epilepsy. CNS Neurol. Disord. Drug Targets 2023, 22, 394–403.
- Löscher, W.; White, H.S. Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments. Cells 2023, 12, 1233.
- Guignet, M.; Campbell, A.; White, H.S. Cenobamate (XCOPRI): Can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia 2020, 61, 2329–2339.
- Binnie, C.D.; Trenité, D.G.; Korte, R. Photosensitivity as a model for acute antiepileptic drug studies. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 35–41.
- Kasteleijn-Nolst Trenite, D.G.A.; DiVentura, B.D.; Pollard, J.R.; Krauss, G.L.; Mizne, S.; French, J.A. Suppression of the photoparoxysmal response in photosensitive epilepsy with cenobamate (YKP3089). Neurology 2019, 93, e559–e567.
- Sommerfeld-Klatta, K.; Zielińska-Psuja, B.; Karaźniewcz-Łada, M.; Główka, F.K. New Methods Used in Pharmacokinetics and Therapeutic Monitoring of the First and Newer Generations of Antiepileptic Drugs (AEDs). Molecules 2020, 25, 5083.
- Oh, J.H.; Jeong, J.W.; Ji, Y.G.; Shin, Y.M.; Lee, K.R.; Cho, K.H.; Koo, T.S. Development of a liquid chromatography-tandem mass spectrometry method for assaying cenobamate in rat plasma. J. Liquid. Chrom. 2019, 41, 992–997.
- Vernillet, L.; Greene, S.A.; Kim, H.W.; Melnick, S.M.; Glenn, K. Mass Balance, Metabolism, and Excretion of Cenobamate, a New Antiepileptic Drug, After a Single Oral Administration in Healthy Male Subjects. Eur. J. Drug. Metab. Pharmacokinet. 2020, 45, 513–522.
- Charlier, B.; Coglianese, A.; Operto, F.F.; Coppola, G.; Grazia, U.; Menna, P.; Filippelli, A.; Piaz, F.; Izzo, V. Development and Validation of a UHPLC-MS/MS-Based Method to Quantify Cenobamate in Human Plasma Samples. Molecules 2022, 27, 7325.
- Arnold, S. Cenobamate: New hope for treatment-resistant epilepsy. Lancet Neurol. 2020, 19, 23–24.
- Steinhoff, B.J. Cenobamate tablets as a treatment for focal-onset seizures in adults. Expert Rev. Clin. Pharmacol. 2021, 14, 161–172.
- Vossler, D.G. Remarkably High Efficacy of Cenobamate in Adults With Focal-Onset Seizures: A Double-Blind, Randomized, Placebo-Controlled Trial. Epilepsy Curr. 2020, 20, 85–87.
- Ferrari, L.; Nisman, A.; Pegan, A.; Ursino, J. An Ex Vivo Evaluation of Cenobamate Administered via Enteral Tubes. Drugs R D 2020, 20, 125–133.
- Löscher, W. Single-Target Versus Multi-Target Drugs Versus Combinations of Drugs with Multiple Targets: Preclinical and Clinical Evidence for the Treatment or Prevention of Epilepsy. Front. Pharmacol. 2021, 12, 730257.
- Steinhoff, B.J. Cenobamate—A new perspective for epilepsy treatment. Nervenarzt 2021, 92, 150–160.
- Nakamura, M.; Cho, J.H.; Shin, H.; Jang, I.S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019, 855, 175–182.
- Sankar, R. Treatment of status epilepticus: Physiology, pharmacology, and future directions. Epilepsia Open 2023, 8, 141–148.
- Sharma, R.; Nakamura, M.; Neupane, C.; Jeon, B.H.; Shin, H.; Melnick, S.M.; Glenn, K.J.; Jang, I.S.; Park, J.B. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur. J. Pharmacol. 2020, 879, 173117.
- Grasshoff, C.; Netzhammer, N.; Schweizer, J.; Antkowiak, B.; Hentschke, H. Depression of spinal network activity by thiopental: Shift from phasic to tonic GABA(A) receptor-mediated inhibition. Neuropharmacology 2008, 55, 793–802.
- Tateiwa, H.; Chintala, S.M.; Chen, Z.; Wang, L.; Amtashar, F.; Bracamontes, J.; Germann, A.L.; Pierce, S.R.; Covey, D.F.; Akk, G.; et al. The Mechanism of Enantioselective Neurosteroid Actions on GABAA Receptors. Biomolecules 2023, 13, 341.
- Roberti, R.; Caro, C.; Iannone, L.F.; Zaccara, G.; Lattanzi, S.; Russo, E. Pharmacology of Cenobamate: Mechanism of Action, Pharmacokinetics, Drug-Drug Interactions and Tolerability. CNS Drugs 2021, 35, 609–618.
- Greene, S.A.; Kwak, C.; Kamin, M.; Vernillet, L.; Glenn, K.J.; Gabriel, L.; Kim, H.W. Effect of cenobamate on the single-dose pharmacokinetics of multiple cytochrome P450 probes using a cocktail approach in healthy subjects. Clin. Transl. Sci. 2022, 15, 899–911.
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717.
This entry is offline, you can click here to edit this entry!
