Pregnancy is accompanied by an increased need for oxygen in the mitochondria of the placenta and a tendency to develop oxidative stress. Oxidative stress represents a disturbance in the balance of oxidation–reduction processes in the body that occurs due to the excessive production of free oxygen radicals that cellular homeostatic mechanisms are unable to neutralize. When the balance with the antioxidant system is disturbed, which happens when free oxygen radicals are in high concentrations, serious damage to biological molecules occurs, resulting in a series of pathophysiological and pathological changes, including cell death. Therefore, oxidative stress plays a significant role in the pathogenesis of many complications that can occur during pregnancy.
- pregnancy
- lipid metabolism
- oxidative stress
1. Introduction
2. Causes of Oxidative Stress in Pregnancy
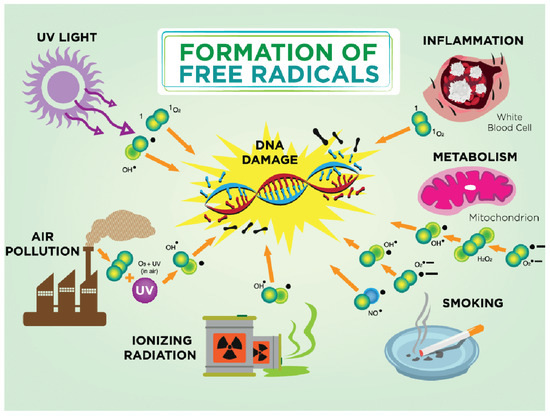
3. Free Radicals
This entry is adapted from the peer-reviewed paper 10.3390/ijms241511964
References
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374.
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860.
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative stress, placental age-ing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653.
- Pospíšil, P.; Prasad, A.; Rác, M. Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 258.
- Kumar, R.; Jafri, M.S. Computational Modeling of Mitochondria to Understand the Dynamics of Oxidative Stress. Methods Mol. Biol. 2022, 2497, 363–422.
- Yin, Y.; Shen, H. Common methods in mitochondrial research (Review). Int. J. Mol. Med. 2022, 50, 126.
- Rottenberg, H.; Hoek, J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 2017, 16, 943–955.
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436.
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15.
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. Subcell. Biochem. 2018, 87, 167–227.
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619.
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928.
- Brand, J.S.; Gaillard, R.; Wes, J.; McEachan, R.R.C.; Wright, J.; Voerman, E.; Felix, J.F.; Tilling, K.; Lawlor, D.A. Associations of maternal quitting, reducing, and continuing smoking during pregnancy with longi-tudinal fetal growth: Findings from Mendelian randomization and parental negative control studies. PLoS Med. 2019, 16, e1002972.
- Pereira, B.; Figueiredo, B.; Pinto, T.M.; Míguez, M.C. Effects of Tobacco Consumption and Anxiety or Depression during Pregnancy on Maternal and Neonatal Health. Int. J. Environ. Res. Public Health 2020, 17, 8138.
- Ioakeimidis, N.; Vlachopoulos, C.; Katsi, V.; Tousoulis, D. Smoking cessation strategies in pregnancy: Current concepts and controversies. Hell. J. Cardiol. 2018, 60, 11–15.
- Langley-Evans, S.C.; Pearce, J.; Ellis, S. Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: A narrative review. J. Hum. Nutr. Diet. 2022, 35, 250–264.
- Lewandowska, M.; Więckowska, B.; Sajdak, S. Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension, and gestational diabetes mellitus. J. Clin. Med. 2020, 9, 1980.
- Zygula, A.; Kosinski, P.; Wroczynski, P.; Makarewicz-Wujec, M.; Pietrzak, B.; Wielgos, M.; Giebultowicz, J. Oxidative Stress Markers Differ in Two Placental Dysfunction Pathologies: Pregnancy-Induced Hypertension and Intrauterine Growth Restriction. Oxid. Med. Cell. Longev. 2020, 2020, 1323891.
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1866, 165354.
- Quan, W.; Zeng, M.; Jiao, Y.; Li, Y.; Xue, C.; Liu, G.; Wang, Z.; Qin, F.; He, Z.; Chen, J. Western Dietary Patterns, Foods, and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Pro-spective Cohort Studies. Adv. Nutr. 2021, 12, 1353–1364.
- Osorio-Yáñez, C.; Gelaye, B.; Qiu, C.; Bao, W.; Cardenas, A.; Enquobahrie, D.A.; Williams, M.A. Maternal intake of fried foods and risk of gestational diabetes mellitus. Ann. Epidemiol. 2017, 27, 384–390.
- Liang, Y.; Gong, Y.; Zhang, X.; Yang, D.; Zhao, D.; Quan, L.; Zhou, R.; Bao, W.; Cheng, G. Dietary Protein Intake, Meat Consumption, and Dairy Consumption in the Year Preceding Pregnancy and During Pregnancy and Their Associations with the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study in Southwest China. Front. Endocrinol. 2018, 9, 596.
- Marí-Sanchis, A.; Díaz-Jurado, G.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Martínez-González, M.A.; Bes-Rastrollo, M. Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: The SUN project. Eur. J. Nutr. 2018, 57, 939–949.
- Li, S.; Tollefsbol, T.O. DNA methylation methods: Global DNA methylation and methylomic analyses. Methods 2021, 187, 28–43.
- Shang, L.; Huang, L.; Yang, L.; Leng, L.; Qi, C.; Xie, G.; Wang, R.; Guo, L.; Yang, W.; Chung, M.C. Impact of air pollution exposure during various periods of pregnancy on term birth weight: A large-sample, retrospective population-based cohort study. Environ. Sci. Pollut. Res. 2021, 28, 3296–3306.
- Shang, L.; Huang, L.; Yang, W.; Qi, C.; Yang, L.; Xin, J.; Wang, S.; Li, D.; Wang, B.; Zeng, L.; et al. Maternal exposure to PM2.5 may increase the risk of congenital hypothyroidism in the offspring: A na-tional database based study in China. BMC Public Health 2019, 19, 1412.
- Smith, R.B.; Fecht, D.; Gulliver, J.; Beevers, S.D.; Dajnak, D.; Blangiardo, M.; Ghosh, R.E.; Hansell, A.L.; Kelly, F.J.; Anderson, H.R.; et al. Impact of London’s road traffic air and noise pollution on birth weight: Retrospective population based cohort study. BMJ (Clin. Res. Ed.) 2017, 359, 5299.
- He, T.; Zhu, J.; Wang, J.; Ren, X.; Cheng, G.; Liu, X.; Ma, Q.; Zhang, Y.; Li, Z.; Ba, Y. Ambient air pollution, H19/DMR methylation in cord blood and newborn size: A pilot study in Zhengzhou City, China. Chemosphere 2018, 212, 863–871.
- Lin, Y.; Zhou, L.; Xu, J.; Luo, Z.; Kan, H.; Zhang, J.; Yan, C.; Zhang, J. The impacts of air pollution on maternal stress during pregnancy. Sci. Rep. 2017, 7, 40956.
- Yang, L.; Xie, G.; Yang, W.; Wang, R.; Zhang, B.; Xu, M.; Sun, L.; Xu, X.; Xiang, W.; Cui, X.; et al. Short-term effects of air pollution exposure on the risk of preterm birth in Xi’an, China. Ann. Med. 2023, 55, 325–334.
- Zhang, Y.; Liu, S.; Wang, Y.; Wang, Y. Causal relationship between particulate matter 2.5 and hypothy-roidism: A two-sample Mendelian randomization study. Front. Public Health 2022, 10, 1000103.
- Mainprize, J.G.; Yaffe, M.J.; Chawla, T.; Glanc, P. Effects of ionizing radiation exposure during pregnancy. Abdom. Radiol. 2023, 48, 1564–1578.
- Shih, B.B.; Farrar, M.D.; Vail, A.; Allan, D.; Chao, M.R.; Hu, C.W.; Jones, G.D.D.; Cooke, M.S.; Rhodes, L.E. In-fluence of skin melanisation and ultraviolet radiation on biomarkers of systemic oxidative stress. Free Radic. Biol. Med. 2020, 160, 40–46.
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176.
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.-M.; Bătrînu, M.-G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572.
- Zhelev, Z.; Georgieva, E.; Lazarova, D.; Semkova, S.; Aoki, I.; Gulubova, M.; Higashi, T.; Bakalova, R. “Redox Imaging to Distinguish Cells with Different Proliferative Indexes: Superoxide, Hydroperoxides, and Their Ratio as Potential Biomarkers. Oxid. Med. Cell Longev. 2019, 2019, 6373685.
