Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Among the current detection methods, technology based on fluorescent probes, with the advantages of sensitivity, convenience, accuracy, cost, and reliability, has recently shown pluralistic applications in the food industry, which is significant to ensure food safety.
- fluorescent probes
- heavy metal detection
- food safety
1. Introduction
Metal elements are widely used in the daily lives by virtue of their good thermal and electrical conductivities, special luster, and excellent ductility. However, with the rapid development of the modern economy, excessive discharge of waste from industry, random abuse of metal-containing pesticides and fertilizers, and improper handling of common daily necessities containing metals have led to the massive discharge of heavy metals as ions into the soil and surface water [1][2]. Heavy metals in the environment can be easily accumulated in aquatic products, crops, and other foodstuffs in the form of ions and then be absorbed into the human body through the food chain, which gradually increases the threat to health via unconscious intaking of heavy metals in the daily lives [3][4].
Typically, heavy metals are considered as inorganic irritant toxicants and are generally classified into three groups: toxic metals (e.g., mercury, lead, chromium, cadmium, nickel, arsenic, cobalt, tin), precious metals (e.g., palladium, platinum, silver, gold, plutonium), and radionuclides (e.g., uranium, thorium, radium, americium) [5]. In particular, mercury, lead, chromium, and cadmium are present at high levels in the environment and accumulated easily in the food chain [6][7]. It has been shown that these heavy metals are prone to react with biomolecular affinity sites and trigger structural changes in biological function molecules in living organisms due to their well-coordinated interactions with biological function molecules containing nitrogen (N), oxygen (O), and sulfur (S), thus affecting various cellular enzymes and protein systems inside the body and disrupting their normal physiological effects [8][9]. For example, mercury (Hg) induces oxidative damage to the mucosa of the gastrointestinal tract and proximal renal tubules, which manifests clinically as abdominal pain, hemorrhagic gastroenteritis, acute tubular necrosis, and subacute shock [10][11]. The pathophysiological toxicity of lead (Pb) is fairly complicated as it involves almost every organ system, with the most severe neurological manifestations being seizures and coma [12][13][14]. Cadmium (Cd) can disrupt iron homeostasis in humans by inducing hyperactivation of heme oxygenase-1 (HO-1) and disrupting lipid metabolism, which ultimately leads to iron apoptosis [15][16][17][18]. The epidemiology of infertility has been shown to be related to the impact of exposure to the heavy metals lead and cadmium [19][20]. Chromium (Cr)-induced ROS-mediated oxidative stress has been disclosed to lead to redox imbalance and affect the balance of antioxidant systems in the body [21]. In conclusion, heavy metals can be uptaken through the respiratory, digestive, and dermal tracts, enter the bloodstream, and rapidly distribute to organs and tissues throughout the body, which ultimately leads to neurological disorders, skin and vascular damage, immune system dysfunction, gastrointestinal and renal dysfunction, and even some cancers. Therefore, the development of rapid, sensitive, and specific-response qualitative and quantitative methods for the detection of heavy metals in food and agriculture-related matrices is of great importance for food safety, environmental monitoring, and clinical diagnosis.
The determination of heavy metals is facilitated by applying novel instrumental methods, such as atomic absorption spectrometry, neutron activation analysis, X-ray fluorescence spectrometry, ion chromatography, Raman spectroscopy, etc., toward the detection of metal elements [22][23][24]. Among them, fluorescent small molecule materials have attracted much attention because of their short response time, simple operation, high selectivity and sensitivity, and greater suitability for the analysis of trace metal ions in complex matrices [25][26] (Scheme 1).
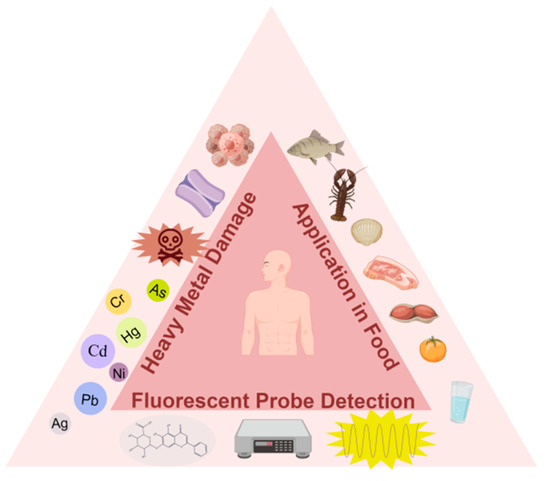
Scheme 1. Fluorescent probe-based assays for determining heavy metals in food (e.g., Cr, Cd, Hg, Pb, Ag, As).
2. Common Fluorescence Spectroscopy Detection Methods
Rapid developments in photochemistry have greatly facilitated the development of instruments and methods for the convenient and accurate detection of metals, quasi-metals, and selected non-metallic elements at (super) trace levels since the 1980s [27]. Common spectroscopic analytical methods include inductively coupled plasma emission spectrometry (ICP-MS), atomic fluorescence spectrometry (AFS), atomic absorption spectrometry (AAS), atomic emission spectrometry (AES), X-ray fluorescence spectrometry (XRF), etc. [22][28]. Further breakthroughs in convenience and sensitivity have been achieved by simplifying sample pre-treatment, combining different analytical methods in recent years, and optimizing analytical conditions, thereby enabling the monitoring of the spatial distribution of heavy metals in food entities [29] (Table 1).
ICP-MS has been reported in the literature as the most effective quantitative analytical method for measuring trace elements in food samples (such as peanuts [30], salted foods, and sea salt [31]), due to its high sensitivity and selectivity. Additionally, it can be combined with other technologies for superior detection efficiency. A new method for the efficient monitoring of the spatial distribution of Hg and Se in mushrooms (with a spatial resolution as low as 5 µm) was proposed for the first time, with researchers using laser ablation (LA) and ICP-MS to quantify mercury and selenium in mushroom substrates with detection limits of 0.006 and 0.3 µg·g−1, respectively [32]. For accomplishing the simultaneous determination of trace selenium and cadmium in rice samples, G. Lan et al. [33] first introduced bypass gas to modify graphite furnace electrothermal evaporation (GF-ETV) and ICP-MS. Through optimization, the detection limits of selenium and cadmium were as low as 0.5 and 0.16 µg·kg−1 respectively, with repeated determinations exhibiting relative standard deviations (RSDs) within 8% (n = 6). Pre-concentration of samples to enhance sensitivity is another focus of improvement schemes. Solid phase extraction (SPE) is the simplest method for pre-concentration. Compared with the classical liquid–liquid extraction method, SPE presents advantages such as less sample consumption, higher multiples of accumulation, better recoveries, rapid phase separation, cheaper costs, etc. Based on these advantages, a method was constructed with flow injection (FI) and SPE-ICP-MS [34]. It combined chemometric methods with experimental design and multi-response surface methodology to simultaneously determine toxic elements. The method successfully reduced the detection limits, ranging from 0.8 ng·L−1 for Mn to 0.09 μg·L−1 for Hg, with relative standard deviations all < 5%, and was successfully applied to the determination of heavy metals in rice and rice products. Similarly, D. Chen et al. [35] established a simple and efficient method, which combined high-performance liquid chromatography–atomic fluorescence spectrometry (HPLC-AFS) and SPE. The method showed high sorption capacity and a wide range of adaptability for the simultaneous determination of four forms of mercury (Hg2+, MeHg, EtHg, PhHg). Under optimized conditions, the limits of detection were 0.001–0.002 µg·L−1, the recoveries were 87.2–111%, and the reproducibilities were 1.1–6.5% in water samples. Pre-treatment of complex samples before detection is another significant scheme to enhance flexibility and sensitivity. For example, pre-emptive microwave digestion of samples, such as beverages and chocolate, utilizing 20% nitric acid (v/v) made it possible to decrease the detection limit to parts per trillion (ng·L−1) using an ICP-MS technique [36]. Similarly, when studying sample pre-treatment methods, such as type, concentrations, and system ratios of ablation reagents, for the combination of ICP-MS and atomic emission spectrometry (AES), a method for the simultaneous determination of Ag, As, Bi, Cd, Cr, and other heavy metals in turmeric was developed [37].
AES, AFS, and AAS are complementary technologies. They have become mainstream methods for determining heavy metals in agriculture and potable water due to their advantages of low detection limits, high accuracy, good selectivity, less sample consumption, and a wide range of applications. They are suitable for the analysis of trace components in samples such as wheat flour [38], honey [39], and milk powder [40]. Two serially-connected graphite tubes were innovatively applied as two electrolytic cells to form an electrochemical vapor generation atomic fluorescence spectrometry (EcHG) system with AES [41]. It worked well in the determination of trace Cd without ion exchange membranes, with easy assembly, direct sample detection, and stable signal retention. The limit of detection was 0.05 ng·mL−1, with a relative standard deviation of 3.2%, and the EcHG efficiency was 38.4 ± 2.2%. Also, it was successfully applied to the determination of potable water samples with recoveries of 95–109%. Coupling with other technologies facilitates further improvements in detection efficiency. A chemical vapor generation multi-channel non-dispersive atomic fluorescence spectrometer (CVG-NDAFS) was developed to simultaneously detect arsenic, antimony, selenium, and mercury in herbal foods and biological standards [42]. The optimized method reduced the detection limits to 0.051, 0.034, 0.050, and 0.0058 ng·mL−1, respectively, with relative standard deviations of 0.42%, 0.74%, 0.97% and 1.0%, respectively. Solid injection electrothermal evaporation atomic absorption spectrometry (SS-ETV) was combined with AAS to determine the cadmium content in chocolate [43]. By optimizing the experimental conditions, the limit of detection was successfully decreased from 150 to 70 pg·g−1, accompanied by a high correlation coefficient (R2 > 0.999). Furthermore, the relative standard deviation of the actual sample detection ranged from 1.5% to 6.4%, indicating a satisfactory level of precision.
XRF is an analytical technique that utilizes the absorption variations in samples of X-rays to determine its composition. It offers several advantages, such as rapid analysis, the ability to analyze a wide range of elements, strong applicability, minimal spectral interference, and a nondestructive nature towards the sample. This method is not only capable of analyzing solid block samples but also provides the means to analyze the composition and thickness of individual layers within multilayer coatings [44]. The majority of detection limits can reach up to 10−6, and when combined with separation and enrichment techniques, this limit can be further enhanced to 10−8. Total reflection X-ray fluorescence analysis (TXRF) was successfully applied in the analysis of heavy metal components in herbal medicines [45]. Based on high definition X-ray fluorescence spectroscopy (HDXRF), a method for the rapid quantification of arsenic (As), cadmium (Cd), nickel (Ni), lead (Pb), tin (Sn), and zinc (Zn) in scallops was developed [46], with detection limits of 0.072, 0.070, 0.502, 0.063, 0.033, and 4.383 mg·kg−1, respectively. The RSD values of precision, reproducibility, and stability assays were found to be less than 10%. In recent years, portable instruments have been successfully developed to further broaden the practical applicability of XRF. G. E. Acquah et al. [47] reported a portable handheld X-ray fluorescence (pXRF) spectrometer to successfully achieve highly accurate and efficient detection of trace heavy metal contaminants (chromium, nickel, and arsenic) in fertilizers. Researchers evaluated the common pXRF method, acid ablation inductively coupled plasma mass spectrometry, and diphenylcarbonyldihydrazide colorimetric methods for the assessment of lead detection in 69 spices worldwide, which helped to reduce lead exposure [48].
Laser-induced breakdown spectroscopy (LIBS) technology has gradually emerged in the technical field of heavy metal detection owing to its rapid detection and green advantages [49]. Q. Zhao et al. [50] brought forth a new idea that constructed a heavy metal content prediction model using near-infrared (NIR) and LIBS spectral data, with simultaneous multi-element detection and prediction accuracy as high as 0.90. The coefficients of determination in the optimal prediction models for Zn, Cu, and Pb were 0.9858, 0.9811, and 0.9460, respectively, and the root mean square errors of prediction were 4.3047, 4.9592, and 8.3881 mg·kg−1, respectively, which provided good reproducibility for the rapid detection of heavy metals in lilies.
3. Spectroscopic Detection Methods Based on Fluorescent Probes
Although traditional methods can achieve accurate quantitative analysis of trace heavy metals in food, they often suffer from the disadvantages of complex operation processes, large amounts of reagents, long analysis times, expensive analytical instruments, and high requirements for professional and technical personnel [51]. In contrast, fluorescent probes can emit fluorescence at certain wavelengths when irradiated by ultraviolet light or visible light, with excellent photophysical properties such as high extinction coefficients, excellent quantum yields, and relatively long emission wavelengths. The characteristics of fluorescent probes can be changed with the environment so as to achieve effective detection of the measured substances with the advantages of good sensitivity, high selectivity, and short response times [52]. In recent years, fluorescent probe-based detection methods for heavy metals have been widely investigated by researchers. The focus of these methods has been the design and synthesis of specific probes with appropriate fluorophores and exploring the potential specific mechanisms. Herein, examples are summarized and classified, such as rhodamine, Schiff base, quinoline, coumarin, azoles, thiourea, tetraphenylene (TPE), thiophene, naphthoimide, etc.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28155689
References
- Xu, L.; Dai, H.; Skuza, L.; Xu, J.; Shi, J.; Wang, Y.; Shentu, J.; Wei, S. Integrated survey on the heavy metal distribution, sources and risk assessment of soil in a commonly developed industrial area. J. Photochem. Photobiol. A Chem. 2022, 236, 113462.
- Zhang, Y.; Song, B.; Zhou, Z. Pollution assessment and source apportionment of heavy metals in soil from lead—Zinc mining areas of south China. J. Environ. Chem. Eng. 2023, 11, 109320.
- Yang, J.; Li, X.; Xiong, Z.; Wang, M.; Liu, Q. Environmental Pollution Effect Analysis of Lead Compounds in China Based on Life Cycle. Int. J. Environ. Res. Public Health 2020, 17, 2184.
- Uddin, M.M.; Zakeel, M.C.M.; Zavahir, J.S.; Marikar, F.; Jahan, I. Heavy Metal Accumulation in Rice and Aquatic Plants Used as Human Food: A General Review. Toxics 2021, 9, 360.
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691.
- Qing, Y.; Li, Y.; Yang, J.; Li, S.; Gu, K.; Bao, Y.; Zhan, Y.; He, K.; Wang, X.; Li, Y. Risk assessment of mercury through dietary exposure in China. Environ. Pollut. 2022, 312, 120026.
- Mielcarek, K.; Nowakowski, P.; Puscion-Jakubik, A.; Gromkowska-Kepka, K.J.; Soroczynska, J.; Markiewicz-Zukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Zmudzinska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food Chem. 2022, 379, 132167.
- Shen, Y.; Nie, C.; Wei, Y.; Zheng, Z.; Xu, Z.-L.; Xiang, P. FRET-based innovative assays for precise detection of the residual heavy metals in food and agriculture-related matrices. Coord. Chem. Rev. 2022, 469, 214676.
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972.
- Majdanik, S.; Potocka-Banas, B.; Glowinski, S.; Luzny, S. Suicidal intoxication with mercury chloride. Forensic Toxicol. 2022, 41, 304–308.
- Chen, B.; Dong, S. Mercury Contamination in Fish and Its Effects on the Health of Pregnant Women and Their Fetuses, and Guidance for Fish Consumption-A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15929.
- Wang, Z.; Huang, X.; Li, J.; Liu, N.; Wei, Q. Lead exposure is non-linearly associated with subclinical myocardial injury in the general population without cardiovascular disease. Front. Inpublichealth 2022, 10, 975413.
- Mielke, H.W.; Gonzales, C.R.; Powell, E.T.; Egendorf, S.P. Lead in Air, Soil, and Blood: Pb Poisoning in a Changing World. Int. J. Environ. Res. Public Health 2022, 19, 9500.
- Hemmaphan, S.; Bordeerat, N.K. Genotoxic Effects of Lead and Their Impact on the Expression of DNA Repair Genes. Int. J. Environ. Res. Public Health 2022, 19, 4307.
- He, Z.; Shen, P.; Feng, L.; Hao, H.; He, Y.; Fan, G.; Liu, Z.; Zhu, K.; Wang, Y.; Zhang, N.; et al. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis. Ecotoxicol. Environ. Saf. 2022, 245, 114123.
- Hong, H.; Xu, Y.; Xu, J.; Zhang, J.; Xi, Y.; Pi, H.; Yang, L.; Yu, Z.; Wu, Q.; Meng, Z.; et al. Cadmium exposure impairs pancreatic beta-cell function and exaggerates diabetes by disrupting lipid metabolism. Environ. Int. 2021, 149, 106406.
- Azarmehr, Z.; Ranji, N.; Khazaei Koohpar, Z.; Habibollahi, H. The effect of N-Acetyl cysteine on the expression of Fxr (Nr1h4), LXRalpha (Nr1h3) and Sirt1 genes, oxidative stress, and apoptosis in the liver of rats exposed to different doses of cadmium. Mol. Biol. Rep. 2021, 48, 2533–2542.
- Zou, H.; Chen, Y.; Qu, H.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Bian, J.; Liu, Z. Microplastics Exacerbate Cadmium-Induced Kidney Injury by Enhancing Oxidative Stress, Autophagy, Apoptosis, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 14411.
- McClam, M.; Liu, J.; Fan, Y.; Zhan, T.; Zhang, Q.; Porter, D.E.; Scott, G.I.; Xiao, S. Associations between exposure to single cadmium, lead, mercury and mixtures and women’s infertility and long-term amenorrhea. Medrxiv-Epidemiology 2022, 1–59.
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. 2022, 29, 62067–62092.
- Chakraborty, R.; Renu, K.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Mirza, A.K.; Vellingiri, B.; Iyer, M.; Dey, A.; Valsala Gopalakrishnan, A. Mechanism of chromium-induced toxicity in lungs, liver, and kidney and their ameliorative agents. Biomed. Pharmacother. 2022, 151, 113119.
- Stortini, A.M.; Baldo, M.A.; Moro, G.; Polo, F.; Moretto, L.M. Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions. Sensors 2020, 20, 6800.
- Bec, K.B.; Grabska, J.; Huck, C.W. Miniaturized NIR Spectroscopy in Food Analysis and Quality Control: Promises, Challenges, and Perspectives. Foods 2022, 11, 1465.
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667.
- Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421.
- Shi, C.T.; Huang, Z.Y.; Wu, A.B.; Hu, Y.X.; Wang, N.C.; Zhang, Y.; Shu, W.M.; Yu, W.C. Recent progress in cadmium fluorescent and colorimetric probes. RSC Adv. 2021, 11, 29632–29660.
- Mai, S.; Gonzalez, L. Molecular Photochemistry: Recent Developments in Theory. Angew. Chem. Int. Ed. 2020, 59, 16832–16846.
- Bogomolov, A. Developing Multisensory Approach to the Optical Spectral Analysis. Sensors 2021, 21, 541.
- Wu, D.; Hu, Y.; Cheng, H.; Ye, X. Detection Techniques for Lead Ions in Water: A Review. Molecules 2023, 28, 3601.
- Chen, L.; Ding, M.; Li, Z.; Li, X.; Deng, L. Determination of macro, micro and toxic element concentrations in peanuts from main peanut producing areas of China by ICP-MS: A pilot study on the geographical characterization. RSC Adv. 2022, 12, 16790–16797.
- Hwang, I.M.; Lee, H.M.; Lee, H.W.; Jung, J.H.; Moon, E.W.; Khan, N.; Kim, S.H. Determination of Toxic Elements and Arsenic Species in Salted Foods and Sea Salt by ICP-MS and HPLC-ICP-MS. ACS Omega 2021, 6, 19427–19434.
- Braeuer, S.; Van Helden, T.; Van Acker, T.; Leroux, O.; Van Der Straeten, D.; Verbeken, A.; Borovicka, J.; Vanhaecke, F. Quantitative mapping of mercury and selenium in mushroom fruit bodies with laser ablation-inductively coupled plasma-mass spectrometry. Anal. Bioanal. Chem. 2022, 414, 7517–7530.
- Lan, G.; Li, X.; Jia, H.; Yu, X.; Wang, Z.; Yao, J.; Mao, X. Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry. Molecules 2022, 27, 8176.
- Londonio, A.; Morzan, E.; Smichowski, P. Simultaneous on-line preconcentration and determination of toxic elements in rice and rice-based products by SPE–ICP–MS: Multiple response optimization. J. Food Compos. Anal. 2022, 107, 104388.
- Chen, D.; Lu, L.; Zhang, H.; Lu, B.; Feng, J.; Zeng, D. Sensitive Mercury Speciation Analysis in Water by High-Performance Liquid Chromatography-Atomic Fluorescence Spectrometry Coupling with Solid-Phase Extraction. Anal. Sci. Sepetember 2021, 37, 1235–1240.
- Catenza, K.F.; Donkor, K.K. Determination of Heavy Metals in Cannabinoid-Based Food Products Using Microwave-Assisted Digestion and ICP-MS. Food Anal. Methods 2022, 15, 2537–2546.
- Zeiner, M.; Soltic, M.; Nemet, I.; Juranovic Cindric, I. Multielement Determination in Turmeric (Curcuma longa L.) Using Different Digestion Methods. Molecules 2022, 27, 8392.
- Pirhadi, M.; Alikord, M.; Tajdar-Oranj, B.; Khaniki, G.J.; Nazmara, S.; Fathabad, A.E.; Ghalhari, M.R.; Sadighara, P. Potential toxic elements (PTEs) concentration in wheat and flour products in Iran: A probabilistic risk assessment. Heliyon 2022, 8, e11803.
- Bereksi-Reguig, D.; Bouchentouf, S.; Allali, H.; Adamczuk, A.; Kowalska, G.; Kowalski, R. Trace Elements and Heavy Metal Contents in West Algerian Natural Honey. J. Anal. Methods Chem. 2022, 2022, 7890856.
- Kiani, A.; Arabameri, M.; Moazzen, M.; Shariatifar, N.; Aeenehvand, S.; Khaniki, G.J.; Abdel-Wahhab, M.; Shahsavari, S. Probabilistic Health Risk Assessment of Trace Elements in Baby Food and Milk Powder Using ICP-OES Method. Biol. Trace Elem. Res. 2022, 200, 2486–2497.
- Liu, S.; Duan, X.; Sun, J. Determination of Cadmium in Water Samples by Electrochemical Hydride Generation Atomic Fluorescence Spectrometry Using Series Graphite Tubes as Electrolytic Cells under Constant Voltage. Anal. Sci. April 2020, 36, 419–423.
- Deng, X.; Li, R.; Deng, S. Determination of the Total Content of Arsenic, Antimony, Selenium and Mercury in Chinese Herbal Food by Chemical Vapor Generation-Four-Channel Non-dispersive Atomic Fluorescence Spectrometry. J. Fluoresc. 2020, 30, 949–954.
- Jia, H.; Li, X.; Lan, G.; Wang, Z.; Feng, L.; Mao, X. Fast Detection of Cadmium in Chocolate by Solid Sampling Electrothermal Vaporization Atomic Absorption Spectrometry and Its Application on Dietary Exposure Risk Assessment. Molecules 2022, 27, 6197.
- Pushie, M.J.; Sylvain, N.J.; Hou, H.; Hackett, M.J.; Kelly, M.E.; Webb, S.M. X-ray fluorescence microscopy methods for biological tissues. Metallomics 2022, 14, mfac032.
- Winkler, A.; Rauwolf, M.; Sterba, J.H.; Wobrauschek, P.; Streli, C.; Turyanskaya, A. Total reflection X-ray fluorescence analysis of elemental composition of herbal infusions and teas. J. Sci. Food Agric. 2020, 100, 4226–4236.
- Wang, Y.; Dong, S.; Xiao, J.; Hu, Q.; Zhao, L. A Rapid and Multi-Element Method for the Determination of As, Cd, Ni, Pb, Sn, and Zn in Scallops Using High Definition X-Ray Fluorescence (HDXRF) Spectrometry. Food Anal. Methods 2022, 15, 2712–2724.
- Acquah, G.E.; Hernandez-Allica, J.; Thomas, C.L.; Dunham, S.J.; Towett, E.K.; Drake, L.B.; Shepherd, K.D.; McGrath, S.P.; Haefele, S.M. Portable X-ray fluorescence (pXRF) calibration for analysis of nutrient concentrations and trace element contaminants in fertilisers. PLoS ONE 2022, 17, 1–20.
- Lopez, A.M.; Nicolini, C.M.; Aeppli, M.; Luby, S.P.; Fendorf, S.; Forsyth, J.E. Assessing Analytical Methods for the Rapid Detection of Lead Adulteration in the Global Spice Market. Environ. Sci. Technol. 2022, 56, 16996–17006.
- Liu, F.; Ye, L.; Peng, J.; Song, K.; Shen, T.; Zhang, C.; He, Y. Fast Detection of Copper Content in Rice by Laser-Induced Breakdown Spectroscopy with Uni- and Multivariate Analysis. Sensors 2018, 18, 705.
- Zhao, Q.; Yu, Y.; Hao, N.; Miao, P.; Li, X.; Liu, C.; Li, Z. Data fusion of Laser-induced breakdown spectroscopy and Near-infrared spectroscopy to quantitatively detect heavy metals in lily. Microchem. J. 2023, 190, 108670.
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, D.; Xu, X.; Zhang, Y.; Lin, H. Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Microporous Mesoporous Mater. 2018, 270, 258–264.
- Wagh, S.B.; Maslivetc, V.A.; La Clair, J.J.; Kornienko, A. Lessons in Organic Fluorescent Probe Discovery. Chembiochem 2021, 22, 3109–3139.
This entry is offline, you can click here to edit this entry!
