Adipose tissue is a crucial organ in energy metabolism and thermoregulation. Adipose tissue phenotype is controlled by various signaling mechanisms under pathophysiological conditions. Type II transmembrane serine proteases (TTSPs) are a group of trypsin-like enzymes anchoring on the cell surface. These proteases act in diverse tissues to regulate physiological processes, such as food digestion, salt-water balance, iron metabolism, epithelial integrity, and auditory nerve development. More recently, several members of the TTSP family, namely, hepsin, matriptase-2, and corin, have been shown to play a role in regulating lipid metabolism, adipose tissue phenotype, and thermogenesis, via direct growth factor activation or indirect hormonal mechanisms. In mice, hepsin deficiency increases adipose browning and protects from high-fat diet-induced hyperglycemia, hyperlipidemia, and obesity. Similarly, matriptase-2 deficiency increases fat lipolysis and reduces obesity and hepatic steatosis in high-fat diet-fed mice. In contrast, corin deficiency increases white adipose weights and cell sizes, suppresses adipocyte browning and thermogenic responses, and causes cold intolerance in mice. These findings highlight an important role of TTSPs in modifying cellular phenotype and function in adipose tissue.
1. Introduction
Adipose tissue is an essential organ in energy balance, metabolic homeostasis, and thermogenesis [
1,
2]. There are two major types of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT), which serve distinct functions; WAT is primarily for energy storage, whereas BAT is for thermogenesis. The cellular components and activities in adipose tissue are closely regulated by local and systemic signaling mechanisms. Abnormalities in adipose tissue function can lead to metabolic disorders, e.g., obesity and type 2 diabetes, which are major risk factors leading to cardiovascular disease.
Serine proteases are a class of proteolytic enzymes that modify protein structure and function [
3]. These proteases act in a wide range of tissues to control physiological processes, including cell growth, embryonic development, and tissue homeostasis. Dysregulated serine protease activities are major contributing factors in cardiovascular disease, neuronal disease, and cancer. In adipose tissue, serine proteases (e.g., adipsin, kallikreins, and proprotein convertases) participate in the activation of growth factors, hormones, adipokines, neuropeptides, and metalloproteinases, which are of metabolic significance. Serine protease inhibitors, also known as serpins (e.g., plasminogen activator inhibitor 1 and vaspin), also play a role in regulating adipose tissue function and are implicated in obesity and metabolic dysfunction.
2. TTSPs
2.1. Protein Domains and Post-Translational Modifications
Proteolytic cleavage is one of the most common mechanisms in activating or degrading proteins. The cleavage can also generate protein fragments with new functions, thereby contributing to the functional diversity of the proteome. In the human genome, ~2% of genes encode proteolytic enzymes, among which serine proteases are a major class [
8]. TTSPs are a family of trypsin-like serine proteases. In humans, the TTSP family has 17 members, all of which have a single-span transmembrane segment near the N-terminus and a trypsin-like serine protease domain at the C-terminus [
4,
5]. Based on protein modules between the transmembrane segment and the protease domain, the TTSP family can be divided into four subgroups: the human airway trypsin-like protease (HAT) subgroup, including HAT, HAT-like (HAT-L) 2-5, TMPRSS11A (transmembrane protease serine 11A), and DESC1 (differentially expressed in squamous cell carcinoma 1); the hepsin subgroup, including hepsin, TMPRSS2-5, enteropeptidase, and MSPL (mosaic serine protease large-form); the matriptase subgroup, including matriptase, matriptase-2-3, and polyserase-1; and the corin subgroup with corin only.
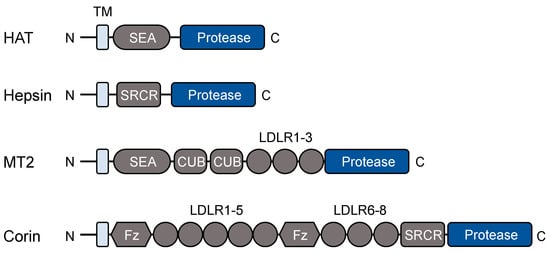
Figure 1 shows domain combinations in selected TTSPs representing each of the four subgroups.
Figure 1. Protein modules in selected TTSPs. All TTSPs contain an N-terminal transmembrane (TM) domain and a C-terminal trypsin-like protease domain. Protein modules between the transmembrane domain and the protease domain vary in individual TTSPs. HAT, human airway trypsin-like protease; SEA, sea urchin sperm protein, enteropeptidase, and agrin; SRCR, scavenger receptor cysteine-rich; MT2, matriptase-2; CUB, complement factor C1s/C1r, urchin embryonic growth factor, and bone morphogenetic protein; LDLR, low-density lipoprotein receptor; Fz, frizzled.
TTSPs are synthesized as zymogens, which are activated by proteolytic cleavage at a conserved site before the C-terminal protease domain. To date, distinct molecular and cellular mechanisms have been identified in TTSP zymogen activation. For example, some TTSPs are activated via autocatalysis either intracellularly, e.g., TMPRSS2 [
9], TMPRSS11A [
10], and TMPRSS13 [
11], or on the cell surface, e.g., hepsin [
12] and matriptase-2 [
13].
N-glycosylation is a common post-translational modification important in protein folding, intracellular trafficking, and cell surface expression [
24,
25]. It has been shown that N-glycans at distinct sites in TTSPs interact with calnexin, an endoplasmic reticulum (ER) chaperone which facilitates glycoprotein folding, quality control, and ER exiting [
26]. Abolishing selected N-glycosylation sites in TTSPs prevents the interaction with calnexin, resulting in ER retention of poorly folded proteins and triggering the unfolded protein response and ER stress [
26].
2.2. Physiological Functions
Based on studies in cells, knockout (KO) mice, and human genetics, physiological functions of many TTSPs have been reported. For example, enteropeptidase is known for trypsinogen activation in food digestion [
42]. Matriptase is essential for dermal and intestinal epithelial integrity [
20,
43,
44,
45,
46,
47]. TMPRSS3 is required for cochlear hair cell survival and normal hearing [
48,
49,
50]. HAT (also known as TMPRSS11D) regulates bronchial epithelial function, including cell proliferation, adhesion, and mucin production [
51,
52,
53,
54]. TMPRSS11A is important in skin wound healing [
55]. TMPRSS13 and HAT-L4 are critical for skin barrier function [
56,
57,
58]. The function of hepsin [
59,
60], matriptase-2 [
61,
62], and corin [
40,
63] in liver function, iron metabolism, and cardiovascular biology, respectively, will be discussed in following sections.
3. TTSP Expression in Adipose Tissues
Adipose tissue is an endocrine organ secreting many adipokines and hormones of metabolic importance [
2]. In adipose tissue, there are many cell populations, including adipogenic progenitors, adipocytes, fibroblasts, immune cells, and vascular cells. Multiple single-cell and spatial transcriptomic studies, together with proteomic analysis, have identified distinct gene profiles in specific cell types [
76,
77]. Amazingly, ~70% of protein-coding genes in the human genome are expressed in adipose tissue, an indication of dynamic biological activities [
78,
79]. The gene-profiling studies provide important insights into the gene and cellular landscapes of human adipose tissue.
Analysis of the Human Protein Atlas database (
www.proteinatlas.org) indicates that many genes in adipose tissue encode serine proteases, metalloproteinases, and cysteine proteases. Among them, several TTSP-encoding genes, including
HPN (encoding hepsin),
ST14 (encoding matriptase),
TMPRSS3,
TMPRSS5, and
TMPRSS11E, are detected. Since all TTSPs contain a cell membrane-anchoring domain, the detected TTSPs are expected to function in adipose tissue. To date, the role of matriptase, TMPRSS3, TMPRSS5, and TMPRSS11E in adipose tissue remains to be defined.
4. Hepsin in Adipose Tissue Differentiation
4.1. Hepsin Protein and Function
Hepsin was discovered in human hepatoma cells [
80]. Based on the cDNA sequence, hepsin was predicted to contain an N-terminal transmembrane domain and an extracellular region with a scavenger receptor cysteine-rich (SRCR) domain and a C-terminal trypsin-like protease domain (
Figure 1).
Consistent with its abundant expression in the liver, the primary function of hepsin is to regulate hepatocyte morphology and function. In hepsin KO mice, hepatocytes are larger in size and sinusoidal capillaries are narrower, compared to those in wild-type (WT) mice [
60,
82]. Hepsin-deficient mice also have reduced levels of triglyceride and glycogen in the liver and low levels of triglyceride, cholesterol, free fatty acids, and albumin in plasma [
83,
84].
The hepatic function of hepsin is mediated, at least in part, by a hepatocyte growth factor (HGF)-dependent mechanism [
83]. HGF is a ligand for c-Met, a key tyrosine kinase receptor in cell differentiation [
86]. HGF is synthesized as a precursor, i.e., pro-HGF, which is activated on the cell surface by proteolytic cleavage.
4.2. Role of Hepsin in Adipose Tissue Browning
As indicated in single-cell sequencing and proteomic analysis, hepsin is expressed in human adipose tissue [
78,
79]. Hepsin mRNA and protein were also detected in mouse WAT and BAT [
83]. Compared to those in WT mice, WAT and BAT weights were lower and adipocyte sizes were smaller in hepsin KO mice, an indication of altered adiposity [
83]. In molecular studies, high levels of brown adipocyte markers, such as uncoupling protein 1 (Ucp1) and cell death-inducing DFFA-like effector A (Cidea), were found in hepsin KO BAT and WAT [
83]. Additionally, levels of beige adipocyte markers (Cd137 and T-box protein 1) in WAT and mitochondrial gene expression, such as
Cox7a1 (encoding cytochrome c oxidase subunit 7A1),
Cpt1b, and
Cpt2 (encoding carnitine palmitoyltransferase 1b and 2, respectively), were increased in hepsin KO BAT and WAT [
83]. These results indicate enhanced adipose tissue browning in hepsin KO mice.
Consistently, cultured interscapular BAT and inguinal WAT from hepsin KO mice were more active in glucose uptake [
83], an indication of increased metabolism. Indeed, hepsin KO mice exhibited a high metabolic rate, as indicated by increased food and water intakes, core body temperature, heat generation, elevated O
2 consumption, CO
2 generation, and respiratory exchange ratio [
83]. In contrast, no changes in motor activities were observed in hepsin KO mice. Unlike WT mice, hepsin KO mice were protected from developing hyperlipidemia and obesity on a high-fat diet. Hepsin deficiency also reduced diabetes and obesity in
db/
db mice [
83]. These data show that hepsin is an important regulator in adipose tissue phenotype and function in mice.
4.3. Regulation of Hepsin Expression in Adipose Tissue
In prostate cancer,
HPN is one of the most highly upregulated genes. Similar
HPN upregulation occurs in breast, ovarian, liver, and stomach cancers [
7]. Oncogenic Ras has been identified as a stimulator in hepsin expression via Raf-MEK-ERK signaling [
94]. In normal tissues, the regulation of
HPN expression is poorly understood. In WT mice on a high-fat diet, no
Hpn expression changes were observed in the liver or adipose tissue [
83]. In contrast, reduced
Hpn mRNA levels were found in the liver and adipose tissue in
db/
db mice [
83]. In WT mice exposed to cold (4 °C), elevated
Hpn expression was noticed in the liver and adipose tissue [
83]. These findings indicate that genetic and environmental factors may play a role in regulating hepsin function.
5. Matriptase-2 in Iron Metabolism and Adiposity
5.1. Matriptase-2 in Iron Metabolism
Matriptase-2 is a hepatic TTSP encoded by the
TMPRSS6 gene in humans [
95]. The protein consists of an N-terminal cytoplasmic segment, a transmembrane domain, and an extracellular region with a SEA (sea urchin sperm protein, enteropeptidase, and agrin) domain, two CUB (complement factor C1s/C1r, urchin embryonic growth factor, and bone morphogenetic protein) domains, three low-density lipoprotein receptor (LDLR) class-A repeats, and a C-terminal trypsin-like protease domain [
95] (
Figure 1). In hepatocytes, matriptase-2 is synthesized as a zymogen, which is activated on the cell surface by autocatalysis [
13]. The primary function of matriptase-2 is to regulate iron metabolism by suppressing hepcidin, a hormone that downregulates the iron transporter ferroportin [
61,
95].
In humans, hepcidin is encoded by the
HAMP gene, which is upregulated by bone morphogenetic protein (BMP) signaling [
98]. In hepatocytes, the binding of BMP to its cell surface receptors activates downstream signaling, thereby increasing
HAMP expression [
99]. Hemojuvelin, a co-receptor for BMP in
HAMP induction, was identified as a main target of matriptase-2 in hepatocytes [
100]. Proteolytic cleavage of hemojuvelin by matriptase-2 reduces BMP signaling and hence hepcidin expression.
5.2. Matriptase-2 in Lipolysis and Obesity
Dysregulated iron metabolism and hepcidin expression are associated with obesity, type 2 diabetes, and insulin resistance. For example, hypoferremia, a deficiency of iron in the circulating blood, occurs more frequently in obese individuals [
105]. High levels of serum hepcidin and poor iron absorption are also common in obese children and women [
106,
107]. In obese patients, hepcidin expression is increased in visceral and subcutaneous adipose tissue where levels of inflammatory proteins, e.g., IL-6 and C-reactive protein, are high [
108].
Given its function in inhibiting hepcidin expression, matriptase-2 may also play a role in regulating metabolism in adipose tissue. In supporting this hypothesis, Tmprss6 KO mice, which had high levels of hepcidin and low levels of plasma iron, were resistant to high-fat diet-induced obesity and hepatic steatosis, as indicated by less total body fat mass, reduced adipocyte sizes and weights in WAT and BAT, and diminished lipid deposits and triglyceride contents in the liver [
110].
The mechanism underlying the protective role of matriptase-2 deficiency against obesity and hepatic steatosis is not fully understood. In principle, the observed resistance to obesity in high-fat diet-treated Tmprss6 KO mice could be due to high hepcidin levels or low iron levels or both. When hypoferremia—but not high hepcidin levels—in Tmprss6 mice was corrected via iron injection, the protection against obesity remained [
110].
6. Corin in Adipose Tissue Phenotype and Thermogenesis
6.1. Corin in Pro-ANP Processing
Corin was discovered in the heart [
40]. The primary function of corin is to activate atrial natriuretic peptide (ANP), a pleiotropic hormone produced in the heart and, at low levels, in non-cardiac tissues. In the heart, ANP serves as a key component in a hormonal mechanism to control blood volume and pressure by relaxing vessels and increasing renal sodium and water excretion [
111]. Moreover, ANP regulates cardiac morphology, function, and aging [
112,
113]. In the pregnant uterus, ANP promotes decidualization and spiral artery remodeling in an auto/paracrine mechanism critical for fetal growth [
114]. Impaired ANP expression and/or function have been implicated in major cardiovascular diseases, including atrial fibrillation, hypertension, cardiac hypertrophy, heart failure, and pre-eclampsia [
111,
115].
6.2. Role of ANP in Lipid Metabolism in Adipose Tissue
In addition to its function in salt–water balance and blood pressure, ANP enhances cellular activity and lipid metabolism in adipose tissue [
122]. In cultured adipocytes, ANP treatment increases lipolysis by stimulating phosphorylation of the hormone-sensitive lipase and perilipin A, a lipid droplet coating protein that recruits lipases in adipocytes [
123]. This ANP function is mediated by increasing intracellular cGMP generation and subsequent activation of the cGMP-dependent protein kinase I (cGKI). Inhibition of cGKI, but not the cAMP-dependent protein kinase (PKA), reduced ANP-mediated lipolysis in adipocytes [
123]. Consistently, intravenous ANP infusion in humans increased lipid hydrolysis and oxidation in subcutaneous abdominal adipose tissue [
124].
6.3. Role of ANP in Adipose Tissue Browning and Thermogenesis
BAT is essential for non-shivering thermogenesis, a survival response in cold environments [
1]. ANP has been shown to promote the browning thermogenic program in human and mouse adipocytes [
130]. In ANP-treated adipocytes, levels of UCP1 and mitochondrial gene expression are markedly increased. This function is mediated by a signaling pathway involving protein kinase G (PKG) and p38 mitogen-activated protein kinase (MAPK) [
130,
131], resulting in the activation of the transcriptional co-activator PGC-1α, a key regulator of mitochondrial biogenesis and thermogenesis in BAT [
93].
6.4. Role of ANP in Adipose Tissue Inflammation
In obese individuals, chronic inflammation in adipose tissue contributes to insulin resistance, metabolic dysfunction, and risks of cardiovascular disease [
137,
138]. Inhibition of inflammation has been considered as a therapeutic target in obesity. ANP is anti-inflammatory [
139,
140]. In cultured human subcutaneous adipose tissue, ANP treatment inhibited the expression and secretion of multiple adipokines (e.g., leptin and retinol-binding protein-4) in adipocytes and cytokines (e.g., TNFα, IL-6, and monocyte chemoattractant protein-1) in macrophages [
141], which are implicated in inflammation and metabolic dysfunction.
6.5. Impaired Adipose Tissue Browning and Thermogenesis in Corin KO Mice
Corin is essential for ANP activation. Given the role of ANP in adipose tissue, it is anticipated that corin deficiency may alter adipose tissue phenotype. Indeed, a recent study reported that Corin KO mice had impaired adipocyte browning, as observed in ANP KO mice [
144]. Particularly, Corin KO mice had an increased fat mass/body weight ratio, larger adipocyte sizes in WAT, and reduced Ucp1 and Cidea expression in BAT. Moreover, decreased p38 Mapk phosphorylation, Pgc-1α levels, and mitochondrial gene expression were also observed in BAT from Corin KO mice [
144]. In culture, ANP treatment increased Ucp1 expression in BAT-derived adipocytes from Corin KO mice [
144]. These data indicate that corin deficiency prevents ANP generation, thereby impairing cGKI/hormone-sensitive lipase-mediated lipolysis and the p38 Mapk-Pgc-1α-Ucp1 signaling pathway in adipose tissue browning in mice (
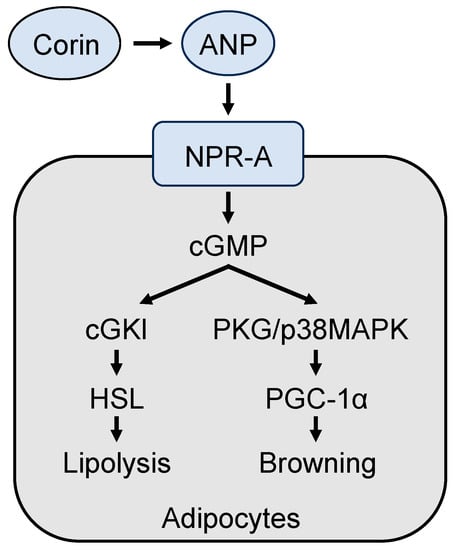
Figure 4).
Figure 4. A model of corin and ANP-mediated mechanisms in promoting lipolysis and browning in adipocytes. Corin activates ANP, which in turn binds to and activates its receptor, natriuretic peptide receptor A (NPR-A), thereby stimulating intracellular cGMP production. Increased cGMP levels promote lipolysis and adipocyte browning via the cGMP-dependent protein kinase I (cGKI)/hormone-sensitive lipase (HSL) pathway and the protein kinase G (PKG)/p38 mitogen-activated protein kinase (MAPK)/PGC-1α pathway, respectively.
7. Conclusions
Adipose tissue is essential in energy metabolism and thermoregulation. Dysregulated adipose function is a major factor in the pathogenesis of metabolic and cardiovascular diseases. Recent studies in mouse models have shown that members of the TTSP family, namely hepsin, matriptase-2, and corin, play an important role in regulating adipose phenotype and thermogenesis via direct signaling or indirect hormonal mechanisms. More studies are expected to verify these findings and to examine if additional underling mechanisms are involved. It will also be important to determine if hepsin, matriptase-2, and corin play similar roles in human adipose tissue and participate in metabolic diseases, such as obesity, dyslipidemia, and type 2 diabetes.
As revealed via single-cell and spatial transcriptomic studies, there are additional TTSPs (e.g., matriptase, TMPRSS3, TMPRSS5, and TMPRSS11E) expressed in human adipose tissue. Further studies are required to determine if these TTSPs act in adipose tissue to regulate cellular components and activities. Findings from such studies shall help to understand the role of TTSPs in adipose tissue function, metabolic homeostasis, and thermogenesis, which are of great biological significance.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11071794