1. Introduction
Aortic aneurysms are permanent dilations of the aortic diameter by more than 50% involving all three layers of the wall [
1]. In the thoracic aorta, progressive dilation and weakening can lead to an aortic dissection/rupture due to an acute tear in the aortic intima leading to the propagation of blood flow through a false lumen. According to the Stanford classification, type A aortic dissections, which involve the ascending thoracic aorta, have a 50% mortality if left untreated, and are far more fatal than type B aortic dissections (which do not involve the ascending or arch aorta), usually managed by medical therapy [
2].
In a large European population-based study, the incidence of aortic dissection was found to be 2.53/100,000 persons/year [
3]. Data from the Global Burden of Disease Study also found that death from aortic aneurysm-related emergencies occurs at a rate of 2.4/100,000 persons, and is the most common cause of death among conditions requiring emergency surgery in high-income countries [
4]. The number of people in the United Kingdom who are 75 or older will double over the next 40 years, and it is estimated that the percentage of incident dissection events in the elderly will rise to 57% by 2050 [
5]. Despite this, size criteria have a low sensitivity, and with current diagnostic methods, it is difficult for clinicians to predict upcoming dissections in patients with thoracic aortopathies.
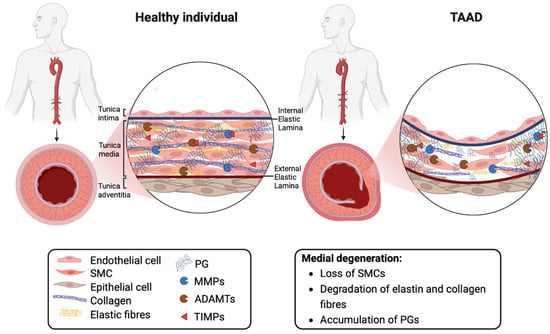
The histopathologic hallmark termed medial degeneration is characterised by smooth muscle cell (SMC) loss, the degradation/disorganisation of elastic and collagen fibres and proteoglycan (PG) accumulation (
Figure 1) [
6]. The net accumulation of PGs in TAAD is presumed to arise from elevated synthesis and reduced proteolytic degradation over a prolonged time period. The proteases responsible for regulating levels of aortic PGs are members of the A Disintegrin-like and Metalloproteinase with Thrombospondin motifs (ADAMTS) family of metalloproteinases [
7].
Figure 1. Extracellular matrix remodelling in the normal aortic wall and in thoracic aortic aneurysms and dissections. Abbreviations: ADAMTS, A Disintegrin-like and Metalloproteinase with Thrombospondin motifs; MMP, matrix metalloproteinase; PG, proteoglycan; SMC, smooth muscle cell; TIMP, tissue inhibitor of metalloproteinase. Image created with BioRender.
2. The Role of ADAMTS Proteoglycanases in Aortic Disease
In humans, the ADAMTS family comprises 19 secreted members. Six of these have been shown to have proteoglycanase activity in vitro: ADAMTS1, 4, 5, 9, 15 and 20 [
7]. Quantitative studies using purified recombinant proteases and full-length versican as a substrate established that ADAMTS5 is the most potent versicanase in vitro, followed by ADAMTS4 and ADAMTS1 [
46,
47], while data for ADAMTS9, 15 and 20 are not currently available.
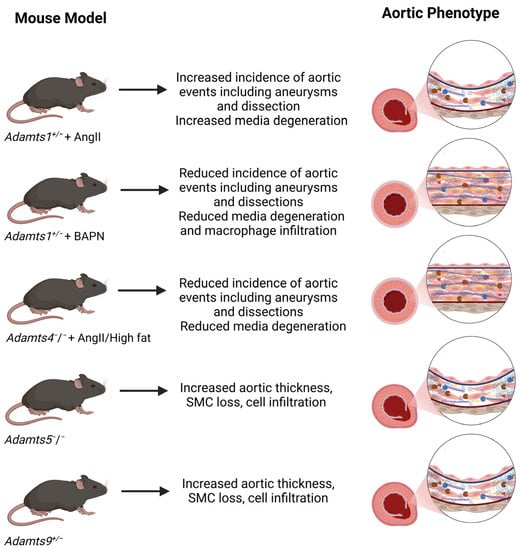
For ADAMTS1, 4, 5 and 9, evidence for a role in aortic development and pathology has emerged predominantly from the phenotype of their respective mouse knockouts models (
Figure 2). Additionally, although ADAMTS19 proteoglycanase activity has not been characterized in vitro, an involvement in PG regulation has been proposed based on the phenotype of
Adamts19 knockout mice as well as the clinical presentation of patients with aortic valve disease [
48,
49].
Figure 2. Aortic phenotype of Adamts knockout mice. Abbreviations: AngII, angiotensin II; BAPN, β-aminopropionitrile. See text for references.
Similar to Fbn1
C1039G/+ mice (a model of MFS), heterozygous
Adamts1+/− mice when subjected to Angiotensin II (AngII) treatment showed an increased incidence of aortic events compared to wild-type mice [
50]. Homozygous
Adamts1 knockout were not investigated in this model, due to elevated perinatal mortality associated with congenital kidney anomalies [
51]. Increased nitric oxide (NO) levels in the aortic wall of
Adamts1+/− mice induced MMP9-dependent elastin fragmentation, aberrant collagen deposition and PG accumulation, three hallmarks of medial degeneration [
50]. The pharmacological inhibition of nitric oxide synthase (NOS2) reversed aortic dilation and protected
Adamts1+/− mice from aortic pathology [
50]. Importantly, patients with MFS showed elevated NOS2 and decreased ADAMTS1 protein levels in their aorta in keeping with the findings in the mice models [
50]. These results suggest that ADAMTS1 expression is required to maintain aortic wall integrity. Interestingly, this function of ADAMTS1 was independent of the TGF-β pathway since losartan administration was not able to reduce aortic dilation or media degeneration induced by
Adamts1 deficiency [
50].
Another model, where β-aminopropionitrile (BAPN), an inhibitor of lysyl oxidase, (the enzyme responsible for cross-linking collagens and elastin) was used to induce TAAD, gave surprisingly different outcomes. When
Adamts1 expression was abrogated post-natally by tamoxifen injection, TAAD incidence and rupture rates were significantly lower than those in non-induced mice (45.5 versus 81.8% and 18.2 versus 42.4%, respectively) [
52]. Medial degeneration and neutrophil/macrophage infiltration were less severe in the
Adamts1−/− mice than in the controls. Decreased inflammation was attributed to the impaired migratory ability of macrophages (in wild-type mice,
Adamts1 expression was found increased predominantly in the adventitia, the major source of inflammatory cells in the aortic wall) [
52].
In mice subjected to AngII treatment and a high fat diet, expression of both
Adamts1 and
Adamts4 increased in aortic SMCs and macrophages [
53].
Adamts4 knockout significantly reduced the incidence of AngII/high fat-induced aortic diameter enlargement, aneurysm formation, dissection and aortic rupture [
53]. This was reflected in the amelioration of aortic media degeneration, with reduced versican and elastic fibre degradation, macrophage infiltration and apoptosis. However, it is difficult to conciliate these findings with the well-known PG accumulation observed in TAAD patients, unless the detrimental function of ADAMTS4 is not directly linked to its proteoglycanase activity. That this is the case is suggested by decreased SMC apoptosis observed in the aortas of
Adamts4−/− mice and in human aortic SMCs transfected with
ADAMTS4 siRNA, thus pointing to a pro-apoptotic role of this protease [
53]. Moreover, ADAMTS4 is present in the atherosclerotic and macrophage-rich areas of the aorta and may be involved in the inflammatory processes of the aortic wall which further degrade ECM components [
7].
Although known for its role in osteoarthritis pathology [
54], ADAMTS5 is also implicated in maintaining normal versican levels, a function important during cardiac valve development and trabeculation [
7]. Similar to
Adamts1+/− mice [
50],
Adamts5−/− mice showed increased aortic dilation in the AgII model, associated with the increased expression of versican and reduced versican proteolysis [
55]. In
Adamts5−/− mice, increased ADAMTS1 levels had little effect on versican cleavage [
7], thus supporting the in vitro data showing that ADAMTS1 has a 1000-fold lower versicanase activity than ADAMTS5 [
47]. During development,
Adamts5−/− mice display a range of ascending aortic anomalies such as SMC loss, cell infiltration and increased aortic thickness, predominantly as a result of aggrecan accumulation [
56]. These are also observed in
Adamts9+/− mice with high penetrance (73%) [
57], suggesting that ADAMTS5 and ADAMTS9 cooperate to maintain a functional ECM during aortic development.
Thus far, genome-wide association studies have not associated variants in
ADAMTS1,
ADAMTS4,
ADAMTS5 or
ADAMTS9 with TAAD. Whole exome sequencing in two unrelated families with consanguineous parents identified four individuals with early onset aortic valve disease that carried two rare homozygous loss-of-function
mutations in
ADAMTS19 [
49]. This prompted the generation of
Adamts19 knockout mice to investigate the role of ADAMTS19 and confirm causality. These mice were viable and fertile but developed progressive aortic valve disease characterized by regurgitation and/or aortic stenosis with 38% penetrance, associated with increased cellularity, PG deposition and ECM disorganization in the valves [
49]. As mentioned above, thus far, ADAMTS19 has not been characterized for its proteoglycanase activity in vitro, and therefore it remains to be ascertained if the observed PG accumulation observed in the aortic valves was directly related to the loss of
Adamts19. Aortic anomalies were not described in these
Adamts19−/− mice. Furthermore, three novel loss of function
ADAMTS19 variants were identified in six individuals from three families who were affected by heart valve disease [
48]. These patients showed a mild dilation in the ascending aorta, in addition to anomalies in their aortic and pulmonary valves.
BAV is a prevalent congenital cardiac anomaly that predisposes individuals to thoracic aortic aneurysm [
58]. It occurs in approximately 2% of the general population, with males representing the majority, accounting for approximately 70% of BAV cases [
58]. Notably, Turner syndrome, a genetic disorder characterised by the absence or partial loss of one X chromosome in females, significantly increases the incidence of BAV by at least 50-fold [
59]. This observation suggests that the lack of a second X chromosome predisposes both males and females with Turner syndrome to develop both BAV and TAA, collectively referred to as BAV aortopathy [
59].
Exome analysis of two cohorts of Turner syndrome patients revealed the presence of risk alleles for
TIMP3, the endogenous inhibitor of ADAMTS1, ADAMTS4 and ADAMTS5, associated with low expression levels [
60]. The hemizygosity (presence of only one copy) of
TIMP1, resulting from the absence of a complete second X chromosome, synergistically amplified the risk of BAV and aortopathy, by upregulating the TIMP1 targets MMP2 and MMP9, two MMPs endowed with elastolytic and gelatinase activity [
60]. The consequent imbalance in ECM breakdown due to elevated MMP/TIMP ratios leads to the structural weakening of the aortic wall [
60]. A higher penetrance (75%) of BAV was also observed in
Adamts5−/−; Smad2+/− mice [
61]. Mechanistically, the lack of ADAMTS5 activity has been shown to interfere with TGF-β signalling through decreased Smad2 phosphorylation. It is not clear how to conciliate these findings with the increased susceptibility to BAV observed in Turner patients. In this context, it will be interesting to cross
Timp3−/− with
Adamts5−/− mice to assess the effect of a combined loss of these interactors on BAV penetrance and severity.
A caveat is that the mouse models used in these studies do not fully mirror human TAAD, as the mice present with a mixture of thoracic and abdominal aortic aneurysms and dissections [
53,
62]. On this regard, the BAPN model may resemble human pathology more closely, since dissections are mainly located in the thoracic aorta [
63].
Levels of ADAMTS proteoglycanases in TAAD patients may shed some light on their role in TAAD development.
3. Levels of ADAMTS Proteases in TAAD
Numerous studies have investigated the expression levels of ADAMTS proteoglycanases in TAAD patients, although these must be replicated in larger cohorts and different populations (Table 1). An additional limitation is that different techniques were used to measure protein levels in tissue (immunoblot, which as a technique is intrinsically qualitative and/or semi-quantitative at best) and in plasma/serum (ELISA), thus making it difficult to compare different studies. The quality of the antibodies used for detection may be a cause of concern in the absence of an extensive validation; different antibodies may lead to different results in different studies. The epitope recognised by these antibodies is generally not reported, nor is it known if complex formation with other blood proteins affects antibody detection. Finally, plasma levels may not directly correlate with tissue levels since ADAMTS proteases are ECM-bound. Taking altogether these limitations, this section will try to draw some conclusions based on the current literature.
Table 1. Summary of studies investigating ADAMTS proteoglycanases in TAAD patients.
From a clinical perspective, studies investigating serum/plasma levels are more relevant than those analysing aortic tissues.
Among the three major proteoglycanases, ADAMTS5 protein has been found to be decreased in plasma [
71] and increased in TAAD tissue compared to non-TAAD controls [
66]; its mRNA levels were found to be decreased in two studies [
6,
70]. In a study which included 83 patients, an independent association was established between decreased plasma levels of ADAMTS5 and an increased risk of acute aortic dissection [
71]. Notwithstanding the limitations discussed above, it seems likely that ADAMTS5 protein levels are decreased in TAAD patients, raising the intriguing possibility that the increased PG levels characteristic of medial degeneration are, at least partially, due to decreased PG clearance.
Three studies found increased ADAMTS4 levels in aortic tissues from TAAD patients [
53,
68,
69], while one study also found higher ADAMTS4 levels in the serum of type A acute dissections compared to controls, with a good diagnostic value (sensitivity: 94.59%; specificity: 97.06%) [
69]. However, ADAMTS4 is mainly produced by macrophages and its levels also increase during atherosclerotic lesion development [
7], hence inflammation may represent a confounding factor. ADAMTS4 mRNA levels were found to be either increased [
53] or unchanged compared to controls [
6]. A recent study by Kaufmann et al. identified a low-molecular-weight cyclic peptide with a high selectivity for ADAMTS4 which acted as a molecular magnetic resonance imaging (MRI) probe. The signal strength enabled the prediction of AAA expansion in a mouse model [
72]. Although it will be very useful to extend these studies to TAAD, this is an important step in visualising and quantifying targets of ECM proteins in a non-invasive manner which helps increasing our understanding of the pathophysiological processes behind the disease.
Four studies found increased ADAMTS1 protein levels in the tissues of TAAD patients [
65,
66,
67,
68], one study found decreased protein levels in the tissues of MFS patients [
50] and two studies found increased plasma/serum levels compared to controls [
65,
69]. One study found higher ADAMTS1 levels in the serum of type A acute dissections compared to the controls, with a good diagnostic value (sensitivity: 87.84%; specificity: 97.06%) [
69]. Since a quantitative study has recently shown that ADAMTS1 and 4 proteoglycanase activities are significantly lower than ADAMTS5 [
47], and it is possible that such high ADAMTS1 levels may represent a compensative response by SMCs, possibly elicited by PG accumulation. Among the other proteoglycanases, only ADAMTS9 and ADAMTS20 mRNA levels were investigated in the tissues of TAAD patients, and these were found to be unchanged [
6].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241512135