Up to 20% of patients with ischemic stroke or transient ischemic attack have a prior history of known atrial fibrillation (AF). Additionally, unknown AF can be detected by different monitoring strategies in up to 23% of patients with cryptogenic or non-cardioembolic stroke. However, most studies had substantial gaps in monitoring time, especially early after the index event. Following this, AF rates would be higher if patients underwent continuous monitoring early after stroke, avoiding any gaps in monitoring. The few existing randomized studies focused on patients with cryptogenic stroke but did not focus otherwise specifically on prevention strategies in patients at high risk for AF (patients at higher age or with high CHA2DS2-VASC scores). Besides invasive implantable loop recorders (ILRs), external loop recorders (ELRs) and mobile cardiac outpatient telemetry (MCOT) are non-invasive tools that are commonly used for long-term ECG monitoring in cryptogenic-stroke patients in the ambulatory setting.
- ECG monitoring
- loop recorder
- atrial fibrillation
- stroke
1. Introduction
2. Technologies to Detect and Monitor Atrial Fibrillation
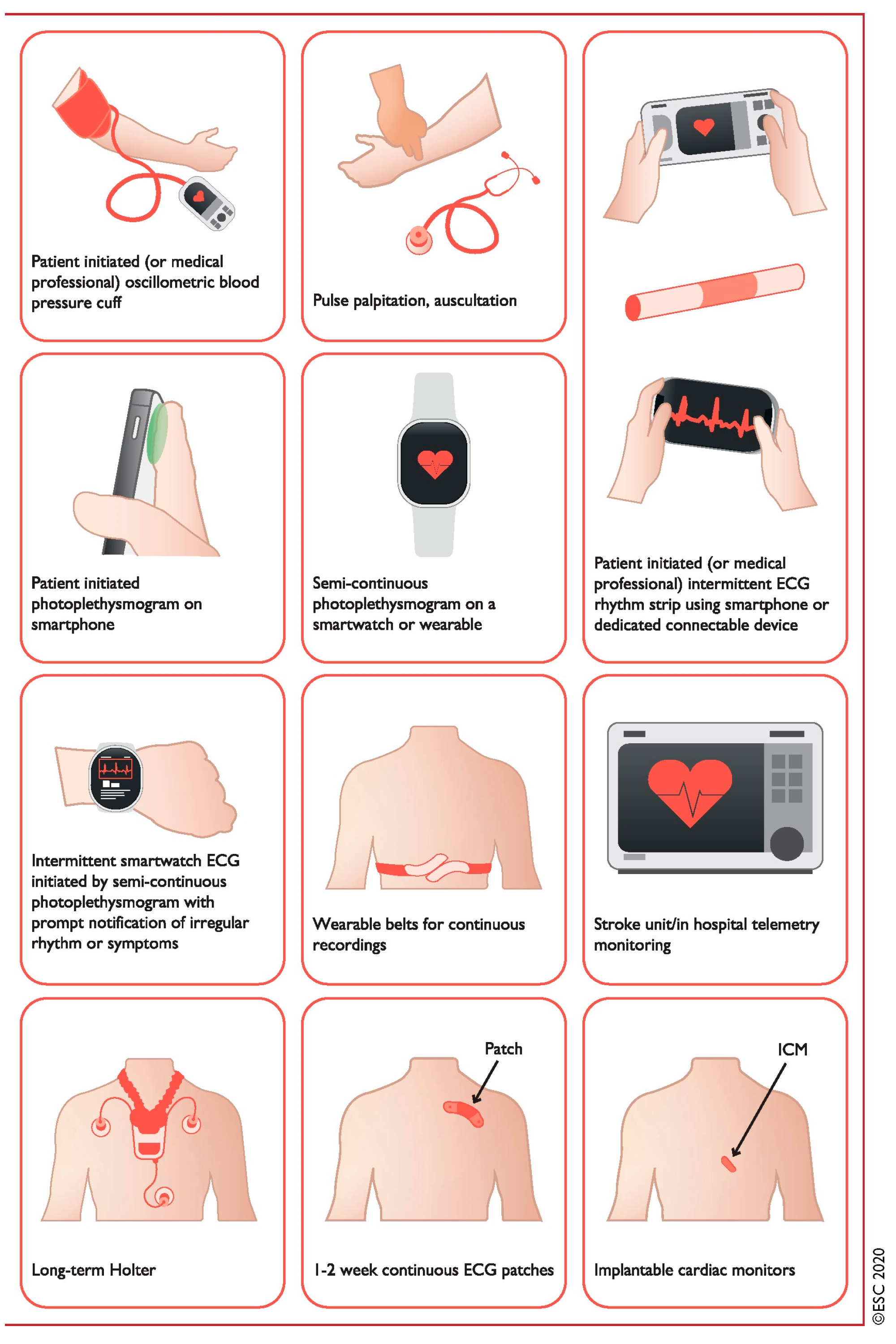
| Method | Pros | Cons |
|---|---|---|
| Noninvasive | ||
| (1) Continuous in-hospital telemetry | - Accurate diagnosis - Detects asymptomatic events |
- Requires inpatient monitoring - Restricts patient movement - Expensive |
| (2) Holter-ECG (24–72 h) (3) Handheld devices |
- Accurate diagnosis - Detects asymptomatic events |
- Short monitoring period - Symptom diary required |
| Patient triggered/event recorder | - Correlation with symptoms - Longer monitoring periods |
- No detection of asymptomatic events - Patient participation required |
| (4) Wearables Mobile automatic/wearable cardiovascular telemetry |
- Continuous recording - Detects asymptomatic events |
- Patient compliance, skin irritation - Expensive |
| Invasive | ||
| (5) Implantable loop recorder | - Follow-up up to 5a - Internet-based data transmission - Detects asymptomatic events - Correlation with symptoms |
- False-positive/-negative detection - Initially expensive and invasive |
| (6) Already implanted PM or ICD | - Endless follow-up - Internet-based data transmission - Detects asymptomatic events - Correlation with symptoms |
- Restricted to small population group |
2.1. Handheld Devices
2.2. Wearable Devices
2.3. Implantable Loop Recorders
2.4. Mobile Platforms and Support Systems
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10070306
References
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Cryptogenic Stroke, E.I.W.G. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014, 13, 429–438.
- Ornello, R.; Degan, D.; Tiseo, C.; Di Carmine, C.; Perciballi, L.; Pistoia, F.; Carolei, A.; Sacco, S. Distribution and Temporal Trends From 1993 to 2015 of Ischemic Stroke Subtypes: A Systematic Review and Meta-Analysis. Stroke 2018, 49, 814–819.
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988.
- Hohnloser, S.H.; Pajitnev, D.; Pogue, J.; Healey, J.S.; Pfeffer, M.A.; Yusuf, S.; Connolly, S.J.; Investigators, A.W. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: An ACTIVE W Substudy. J. Am. Coll. Cardiol. 2007, 50, 2156–2161.
- Yiin, G.S.; Howard, D.P.; Paul, N.L.; Li, L.; Luengo-Fernandez, R.; Bull, L.M.; Welch, S.J.; Gutnikov, S.A.; Mehta, Z.; Rothwell, P.M. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: A population-based study. Circulation 2014, 130, 1236–1244.
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867.
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962.
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486.
- Tsivgoulis, G.; Katsanos, A.H.; Grory, B.M.; Kohrmann, M.; Ricci, B.A.; Tsioufis, K.; Cutting, S.; Krogias, C.; Schellinger, P.D.; Campello, A.R.; et al. Prolonged Cardiac Rhythm Monitoring and Secondary Stroke Prevention in Patients With Cryptogenic Cerebral Ischemia. Stroke 2019, 50, 2175–2180.
- Noubiap, J.J.; Agbaedeng, T.A.; Kamtchum-Tatuene, J.; Fitzgerald, J.L.; Middeldorp, M.E.; Kleinig, T.; Sanders, P. Rhythm monitoring strategies for atrial fibrillation detection in patients with cryptogenic stroke: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2021, 34, 100780.
- Hermans, A.N.L.; Gawalko, M.; Dohmen, L.; van der Velden, R.M.J.; Betz, K.; Duncker, D.; Verhaert, D.V.M.; Heidbuchel, H.; Svennberg, E.; Neubeck, L.; et al. Mobile health solutions for atrial fibrillation detection and management: A systematic review. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2022, 111, 479–491.
- Maines, M.; Zorzi, A.; Tomasi, G.; Angheben, C.; Catanzariti, D.; Piffer, L.; Del Greco, M. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQTM. EP Eur. 2018, 20, 1050–1057.
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Cote, R.; et al. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014, 370, 2467–2477.
- Purerfellner, H.; Pokushalov, E.; Sarkar, S.; Koehler, J.; Zhou, R.; Urban, L.; Hindricks, G. P-wave evidence as a method for improving algorithm to detect atrial fibrillation in insertable cardiac monitors. Heart Rhythm Off. J. Heart Rhythm Soc. 2014, 11, 1575–1583.
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467.
- Edwards, S.J.; Wakefield, V.; Jhita, T.; Kew, K.; Cain, P.; Marceniuk, G. Implantable cardiac monitors to detect atrial fibrillation after cryptogenic stroke: A systematic review and economic evaluation. Health Technol. Assess 2020, 24, 1–184.
- Seet, R.C.; Friedman, P.A.; Rabinstein, A.A. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011, 124, 477–486.
- Chew, D.S.; Rennert-May, E.; Quinn, F.R.; Buck, B.; Hill, M.D.; Spackman, E.; Manns, B.J.; Exner, D.V. Economic evaluation of extended electrocardiogram monitoring for atrial fibrillation in patients with cryptogenic stroke. Int. J. Stroke 2021, 16, 809–817.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498.
- Rozen, G.; Vaid, J.; Hosseini, S.M.; Kaadan, M.I.; Rafael, A.; Roka, A.; Poh, Y.C.; Poh, M.Z.; Heist, E.K.; Ruskin, J.N. Diagnostic Accuracy of a Novel Mobile Phone Application for the Detection and Monitoring of Atrial Fibrillation. Am. J. Cardiol. 2018, 121, 1187–1191.
- Vandenberk, T.; Stans, J.; Mortelmans, C.; Van Haelst, R.; Van Schelvergem, G.; Pelckmans, C.; Smeets, C.J.; Lanssens, D.; De Canniere, H.; Storms, V.; et al. Clinical Validation of Heart Rate Apps: Mixed-Methods Evaluation Study. JMIR Mhealth Uhealth 2017, 5, e129.
- Fan, Y.Y.; Li, Y.G.; Li, J.; Cheng, W.K.; Shan, Z.L.; Wang, Y.T.; Guo, Y.T. Diagnostic Performance of a Smart Device With Photoplethysmography Technology for Atrial Fibrillation Detection: Pilot Study (Pre-mAFA II Registry). JMIR Mhealth Uhealth 2019, 7, e11437.
- O’Sullivan, J.W.; Grigg, S.; Crawford, W.; Turakhia, M.P.; Perez, M.; Ingelsson, E.; Wheeler, M.T.; Ioannidis, J.P.A.; Ashley, E.A. Accuracy of Smartphone Camera Applications for Detecting Atrial Fibrillation: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e202064.
- Hall, A.; Mitchell, A.R.J.; Ashmore, L.; Holland, C. Atrial fibrillation prevalence and predictors in patients with diabetes: A cross-sectional screening study. Br. J. Cardiol. 2022, 29, 8.
- Pezawas, T.; Stix, G.; Kastner, J.; Schneider, B.; Wolzt, M.; Schmidinger, H. Implantable loop recorder in unexplained syncope: Classification, mechanism, transient loss of consciousness and role of major depressive disorder in patients with and without structural heart disease. Heart 2008, 94, e17.
- Guo, Y.; Chen, Y.; Lane, D.A.; Liu, L.; Wang, Y.; Lip, G.Y.H. Mobile Health Technology for Atrial Fibrillation Management Integrating Decision Support, Education, and Patient Involvement: mAF App Trial. Am. J. Med. 2017, 130, 1388–1396.e6.
