Hydrogen is one of the most abundant elements in the universe and ranks as the initial element on the periodic table. Hydrogen storage is one of the most difficult tasks. Hydrogen is kept in special materials and high-pressure tanks, such as those seen in such vehicles as cars and trains. These tanks are not only huge and expensive to construct, but they are also unsuitable for recycling and long-term storage. Because of this, researchers from all around the world are working to find ways to address these limitations and fully use hydrogen. Hydrogen has the advantage of being able to be kept in a variety of forms, including gaseous, liquid, and sometimes solid, despite the fact that storage poses major difficulties.
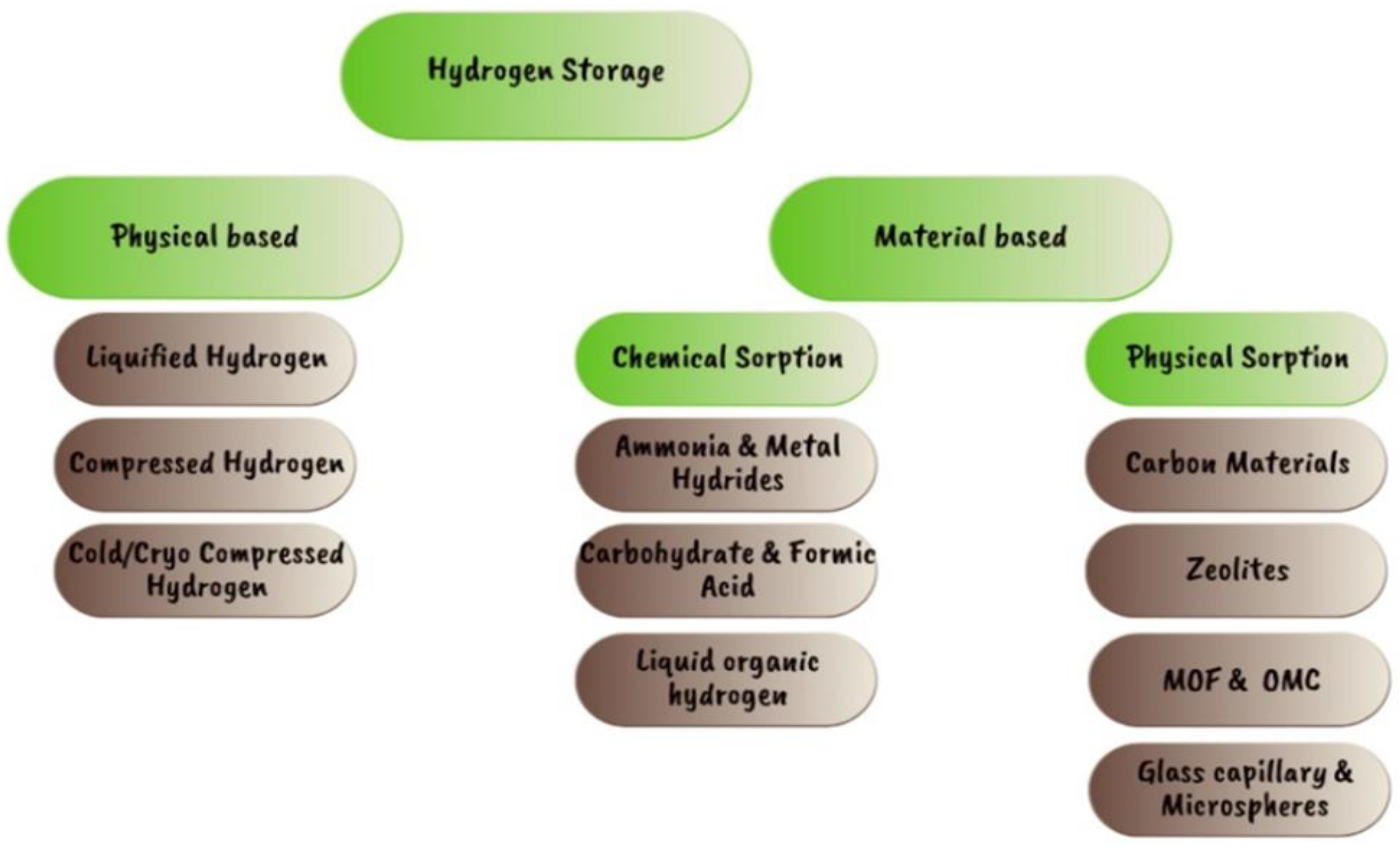
- hydrogen generation
- hydrogen storage
1. Current Hydrogen Storage
|
Materials |
Mechanisms |
Ref |
|---|---|---|
|
Stainless steel |
Hydrogen diffuses into the grain boundaries, mixing with carbon to form methane gas, which boosts system pressure and causes breaking. |
[14] |
|
Carbon steel |
The introduction of hydrogen into the material reduces the material’s micromechanical characteristics. The failure of transition from ductile to brittle occurs. The diffusion of hydrogen into the steel material enhances the combined activities of hydrogen-enhanced localized plasticity and hydrogen-enhanced decohesion. |
[15] |
|
Aluminum and aluminum alloys |
Because of the lower solubility of hydrogen in aluminum at low temperatures, the passage of hydrogen to casting imperfections and drops causes fissures. |
[16] |
|
Copper and copper alloys |
Fissures and blisters form as a result of hydrogen diffusion into oxygen-bearing copper and copper alloys annealed in a hydrogen atmosphere. The copper material’s fracture toughness and ductility are reduced. |
[17] |
|
Nickel and nickel-based alloys |
Hydrogen adsorption at fracture tips of nickel and nickel-based materials accelerates crack propagation in aqueous hydrogen. |
[18] |
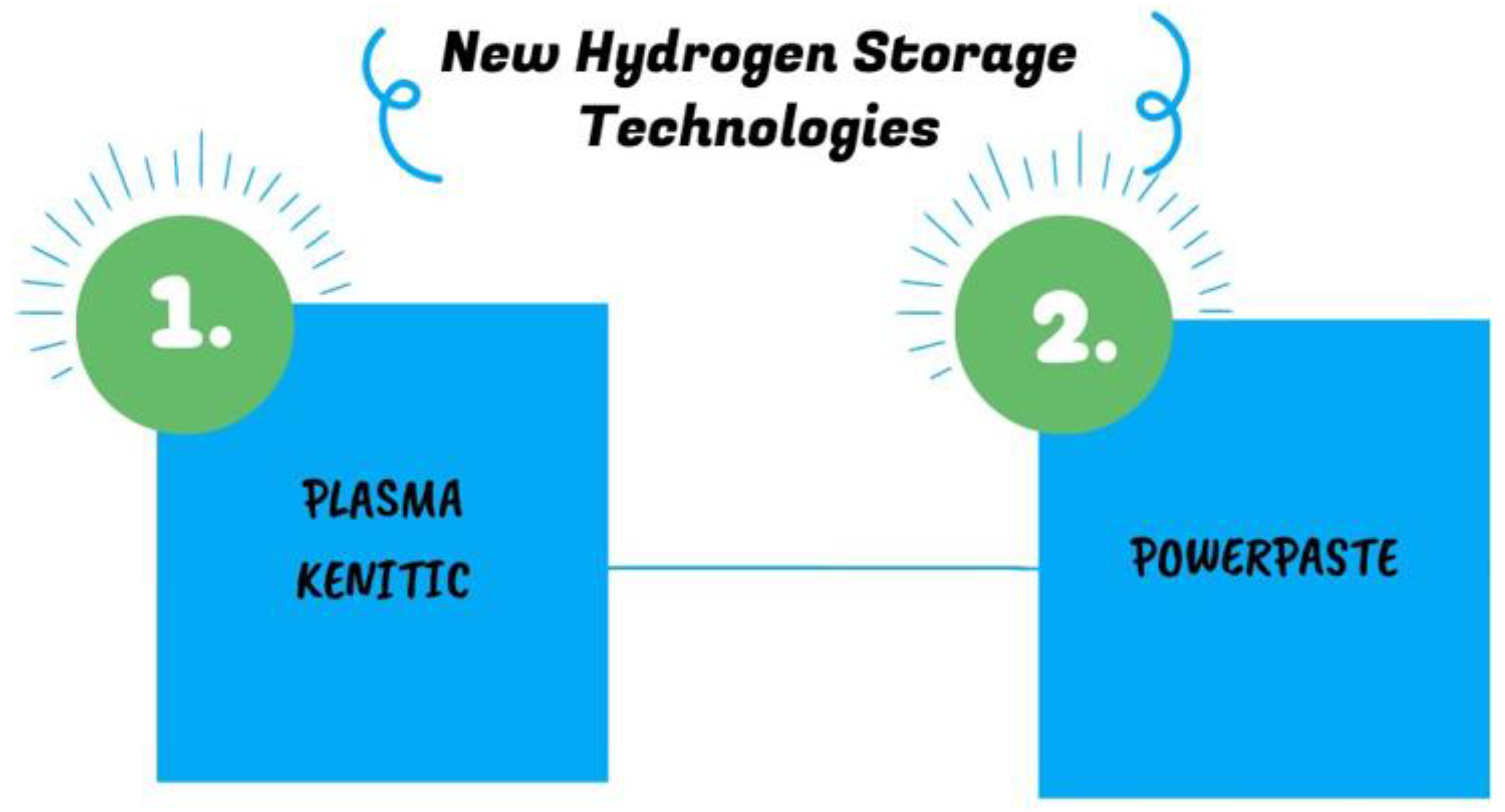
2. Latest Hydrogen Storage Technology
2.1. Plasma Kinetics
2.2. POWERPASTE
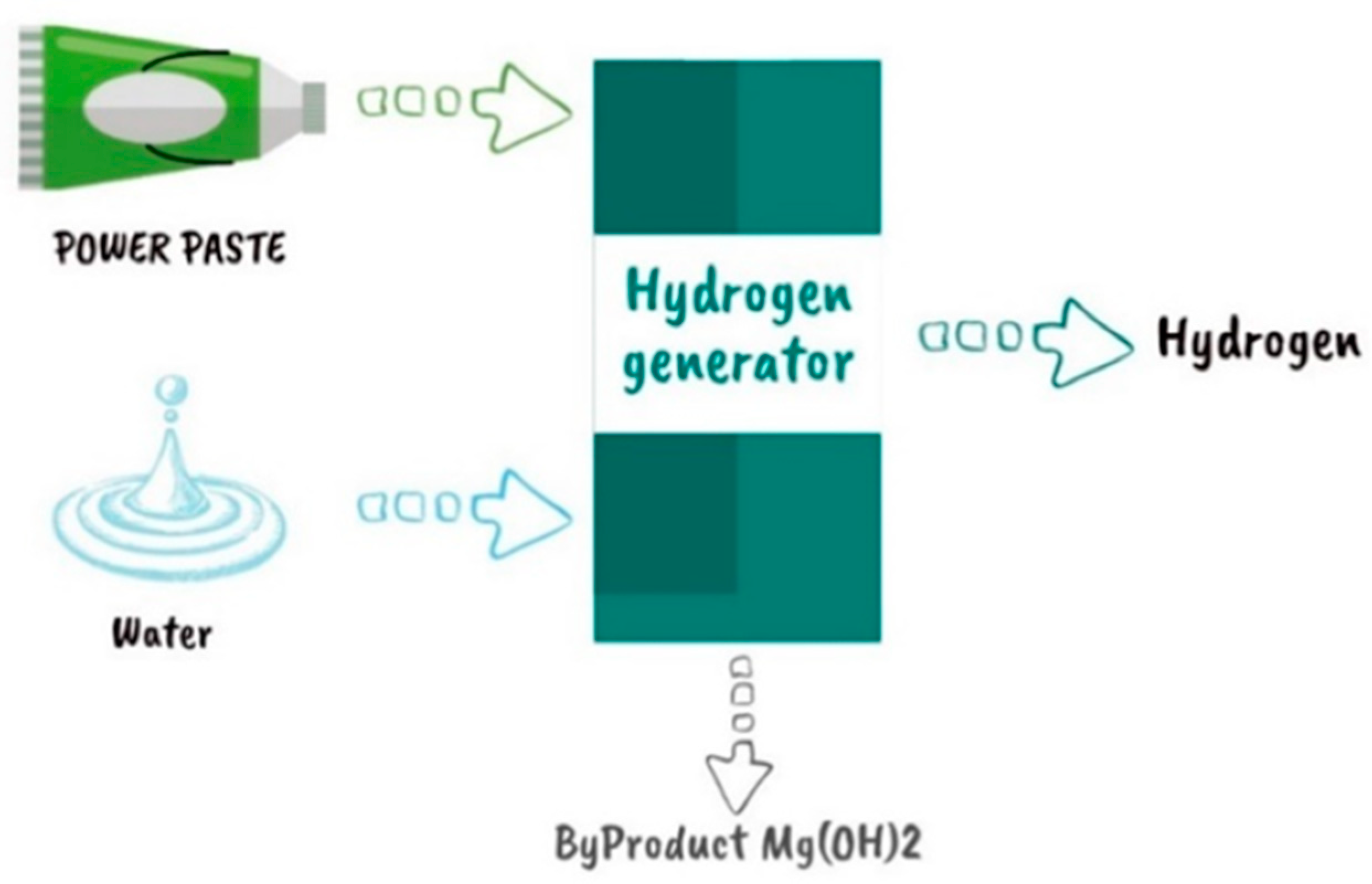
|
Storage/Feature |
Plasma Kinetics |
POWERPASTE |
Compressed |
Liquid |
Metal Hydride |
|---|---|---|---|---|---|
|
Temp/Press stored |
25 °C/1 bar |
Ambient |
25 °C/350–700 bar |
−252.87 °C/1 bar |
+175 °C/20 bar |
|
Energy |
8.7 kWh/kg |
1.6 kWh/kg |
1.8–6.5 kWh/kg |
11.5 kWh/kg |
34.8 kWh/kg |
|
Flammability |
Nonflammable |
Nonflammable |
Flammable |
Flammable |
Flammable |
|
Explosive in air |
Nonexplosive |
Nonexplosive |
Explosive |
Explosive |
Non-explosive |
|
Stored Molecule |
MgHX Hybrid |
MgH2 |
H2 covalent |
H2 covalent |
MgH2 Hybrid |
|
Technology |
Pros |
Cons |
Ref |
|---|---|---|---|
|
Plasma Kinetics |
|
|
[19] |
|
POWERPASTE |
|
|
[20] |
This entry is adapted from the peer-reviewed paper 10.3390/app13158711
References
- Nachtane, M.; Tarfaoui, M.; Abichou, M.A.; Vetcher, A.; Rouway, M.; Aâmir, A.; Mouadili, H.; Laaouidi, H.; Naanani, H. An Overview of the Recent Advances in Composite Materials and Artificial Intelligence for Hydrogen Storage Vessels Design. J. Compos. Sci. 2023, 7, 119.
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269.
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973.
- Nachtane, M.; Tarfaoui, M.; Mohammed, M.A.; Saifaoui, D.; El Moumen, A. Effects of environmental exposure on the mechanical properties of composite tidal current turbine. Renew. Energy 2020, 156, 1132–1145.
- Nachtane, M.; Tarfaoui, M.; Sassi, S.; El Moumen, A.; Saifaoui, D. An investigation of hygrothermal aging effects on high strain rate behaviour of adhesively bonded composite joints. Compos. Part B Eng. 2019, 172, 111–120.
- Lagdani, O.; Tarfaoui, M.; Rouway, M.; Laaouidi, H.; Sbai, S.J.; Dabachi, M.A.; Aamir, A.; Nachtane, M. Influence of Moisture Diffusion on the Dynamic Compressive Behavior of Glass/Polyester Composite Joints for Marine Engineering Applications. J. Compos. Sci. 2022, 6, 94.
- Tarfaoui, M.; Sassi, S.; Lagdani, O.; Nachtane, M. Strain rate effects on the thermomechanical behavior of glass/polyester composite joints. Polym. Compos. 2022, 43, 36–51.
- Nachtane, M.; Meraghni, F.; Chatzigeorgiou, G.; Harper, L.T.; Pelascini, F. Multiscale viscoplastic modeling of recycled glass fiber-reinforced thermoplastic composites: Experimental and numerical investigations. Compos. Part B Eng. 2022, 242, 110087.
- Compatibility of Hydrogen with Different Materials. Available online: https://hyresponder.eu/wp-content/uploads/2021/06/Lecture-4-slides.pdf (accessed on 7 April 2023).
- Zhang, C.; Li, Y.; Xu, X.; Zhang, M.; Leng, H.; Sun, B. Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance. Materials 2023, 16, 2800.
- Birnbaum, H.K. Mechanical Properties of Metal Hydrides. Available online: https://apps.dtic.mil/sti/pdfs/ADA141496.pdf (accessed on 12 July 2023).
- Okonkwo, P.C.; Barhoumi, E.M.; Ben Belgacem, I.; Mansir, I.B.; Aliyu, M.; Emori, W.; Uzoma, P.C.; Beitelmal, W.H.; Akyüz, E.; Radwan, A.B.; et al. A focused review of the hydrogen storage tank embrittlement mechanism process. Int. J. Hydrogen Energy 2023, 48, 12935–12948.
- Cryogenic Safety. Available online: http://www.phys.ufl.edu/courses/phy4550-6555c/spring10/lecture-safety-disasters.pdf (accessed on 12 July 2023).
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723.
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Industrial Components: Prediction, Prevention, and Models. Corrosion 2016, 72, 943–961.
- Safyari, M.; Moshtaghi, M.; Hojo, T.; Akiyama, E. Mechanisms of hydrogen embrittlement in high-strength aluminum alloys containing coherent or incoherent dispersoids. Corros. Sci. 2022, 194, 109895.
- Safyari, M.; Moshtaghi, M.; Kuramoto, S. Environmental hydrogen embrittlement associated with decohesion and void formation at soluble coarse particles in a cold-rolled Al–Cu based alloy. Mater. Sci. Eng. A 2021, 799, 139850.
- Burille, A.; Scheid, A.; Ferreira, D.C.F.; Santana, L.; Kwietniewski, C.E.F. Hydrogen embrittlement of single-phase strain-hardened nickel-based UNS N08830 alloy. Mater. Sci. Eng. A 2021, 803, 140486.
- Plasma Kinetics. Responsible, Renewable Hydrogen Energy Systems. Available online: https://plasmakinetics.com/ (accessed on 27 June 2023).
- POWERPASTE for Off-Grid Power Supply. Available online: https://www.ifam.fraunhofer.de/content/dam/ifam/en/documents/dd/Infobl%C3%A4tter/White_paper_POWERPASTE_final.pdf (accessed on 8 March 2023).