Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In agricultural biogas plants, besides biogas, the by-product digestate is also produced. Due to its high moisture content and organic origin, it can successfully be applied in the hydrothermal carbonization process to avoid the fate of landfilling. The type of feedstock and the parameters of the hydrothermal conversion (HTC) process, such as temperature, pressure and residence time, affects the physical and chemical characteristics of hydrochar. Therefore, its possible application might be as a biofuel, fertilizer, soil improver, adsorber, or catalyst.
- biogas
- digestate
- hydrochar
- hydrothermal carbonization
- biofuel
1. Biogas Production and Fermentation Process
Agricultural biogas plants are installations where biogas is produced. Biogas is a fuel which can be converted into heat or electricity. Biogas is primarily made from organic waste and biomass, so it is classified as a renewable energy source, e.g., wind and solar [1]. Biomass is a renewable energy source, an organic residue from agriculture, forestry and urban development that can be reused with environmental benefits [2]. Agricultural biomass consists mainly of cellulose, hemicellulose, lignin, and lipid extracts. It has a high hydroxyl, carboxyl, and phenolic group content and a low initial carbon content [3]. The most popular method used to obtain biogas is anaerobic digestion. This is a biochemical process where microorganisms convert waste into biofuel. Biogas contains predominantly methane and carbon dioxide but also hydrogen sulphide and nitrogen [4]. To increase the concentration of methane, various purification methods are used.
Water and amine scrubbing, adsorption, and membrane technologies are the most popular. As a result, purified biogas is produced, which is called biomethane [4][5]. Biomethane contains more than 95% of methane. Thus, it can be directly used in industry [6].
Figure 1 depicts a scheme of biogas and digestate production. Firstly, the feedstock is directed into the fermentation chamber. As a result of the anaerobic digestion, biogas and digestate are obtained. The biogas can produce heat, electricity and be purified to become biomethane. Digestate is a solid by-product of biogas production. The proper management of this by-product might be beneficial for the environment. Digestate is a nutrient-rich substance that stimulates the growth of plants. A lack of odour in comparison to initial feedstock, for instance, manure, is another advantage [7]. It consists of organic compounds, macronutrients, and micronutrients. Digestate is mainly built of carbon, nitrogen, phosphorus, potassium, and sodium. The amount of carbon in the digestate indicates the efficiency of the anaerobic digestion process. Nitrogen is a crucial compound if the intention for digestate is used as a fertilizer. Phosphorus is also an essential element in the digestate.
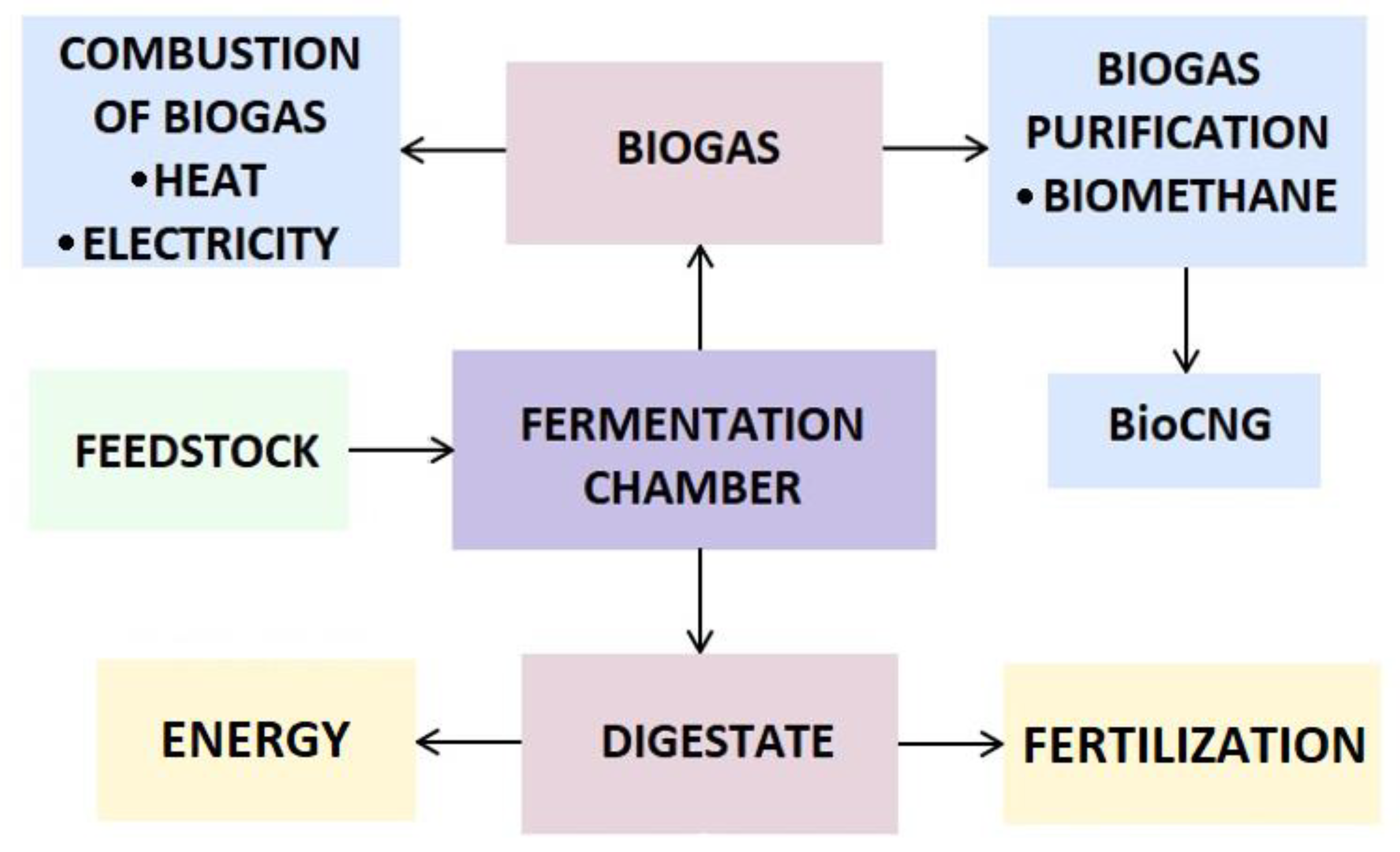
Figure 1. Scheme of biogas and digestate production.
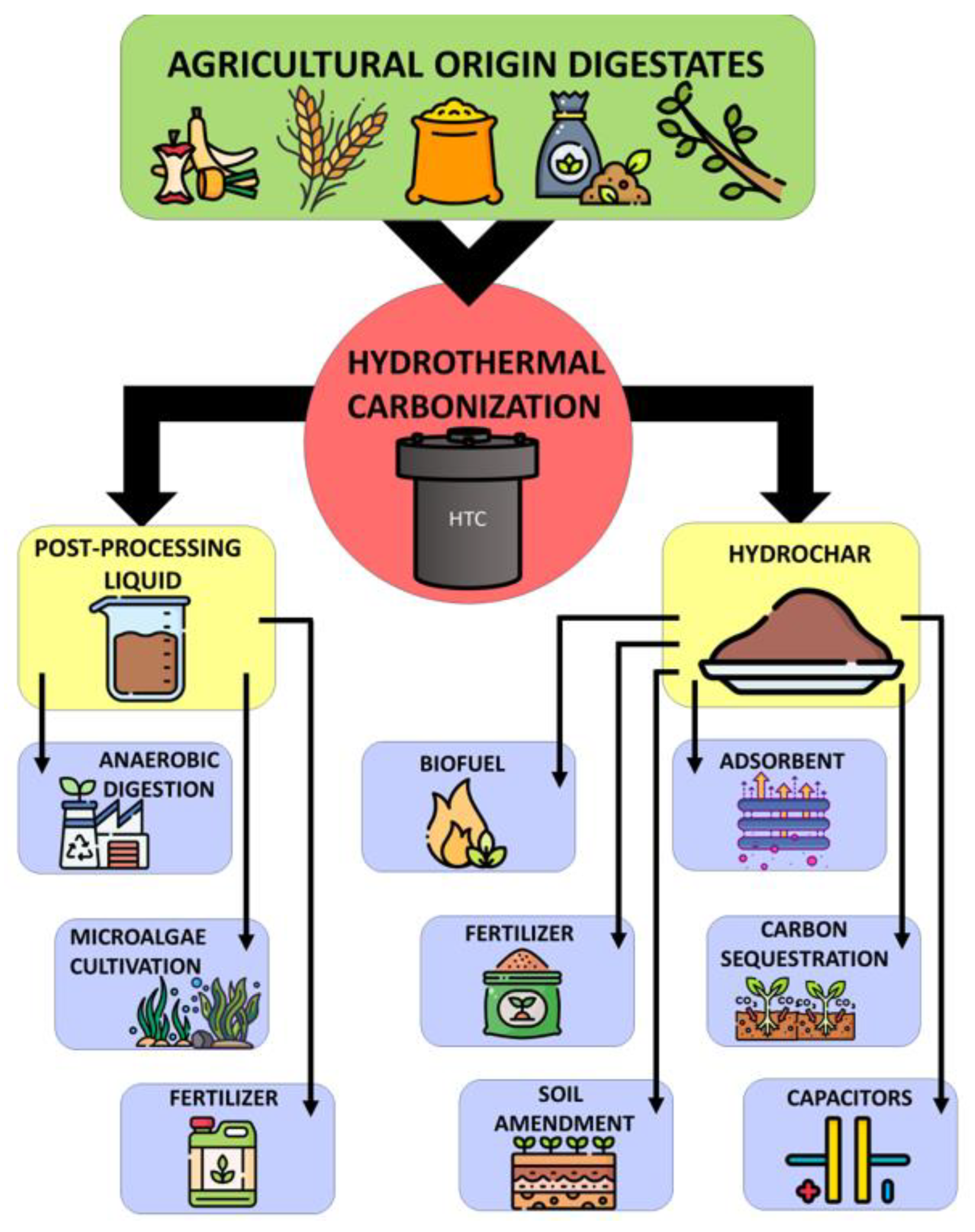
2. Hydrothermal Carbonization of Agricultural Origin Digestates
In the HTC process, the feedstock is heated within a temperature range of 160–280 °C in an aqueous environment and at autogenous pressure. The products of hydrothermal carbonization are solid, liquid, and gaseous phases (Figure 2). The solid fraction is called hydrochar, which can be used as a solid fuel; for the production of syngas, as a fertilizer and soil improver (due to the content of nutrients, e.g., phosphorus, nitrogen, and potassium, it improves soil properties and helps in creating a humus layer) [8]. Hydrochar can also be used as an adsorbent for environmental remediation [9]. The temperature of HTC is the most important parameter responsible for the course of the entire process [10], while the residence time of the process is a key function parameter [9][11][12]. The HTC method does not require drying of the feedstock because the process’s environment is already aquatic. The process is usually applied to organic waste and biomass with high moisture content, up to 85% [9]. The high-water content of digestate is associated with an increase in transport costs and, thus, an increase in expenditure on energy production [11]. It is a very effective method that improves dewaterability and disinfection of various viruses, bacteria, and pathogens [13].

Figure 2. Hydrothermal carbonization products disposal.
The chemical processes of raw materials during HTC are hydrolysis, dehydration, decarboxylation, condensation, polymeric condensation, and aromatization [14][15]. For reactions to occur, the temperature must exceed 100 °C. At 180 °C, substantial hydrolysis takes place. The raw material must be immersed in water, and the pH should be lower than 7 [16]. Hydrolysis occurs in the presence of water and leads to the breakdown of ester and ether bonds of molecules. The rate of the hydrolysis of biomass is influenced by diffusion. Above 200 °C, cellulose hydrolysis takes place, and at 180 °C, hemicellulose [16]. The hydrolysis of lignin occurs above 260 °C because it has more ether bonds [17]. Alkaline conditions were estimated to give the highest reaction rates compared to acidic and neutral conditions. An acidic reaction is essential in the case of a later stage of glucose degradation. The substrates usually contain cellulose and lignin. In the first stage of hydrolysis, glucose is formed from cellulose and phenol from lignin.
Further processes are very complex. Performing the process of glucose dehydration leads to form, e.g., organic acids, ketones, and phenols. Biomass of agricultural origin consists of different lignin, cellulose, and hemicellulose contents. Moreover, there is a likelihood of hemicellulose interacting with lignin. As a result, there may be a better solubility of its aromatic structures. These two components together form an oligomer that is stable under hydrothermal conditions. From oligomers, monomers are formed, from which organic acids are created. Acetic acid affects the gradual lowering of pH and, as a result, it leads to dehydration and decarboxylation. The next stage is dehydration, i.e., removing water from the raw material. This process results in a reduction in H:C and O:C ratios in biomass. The following stages are decarboxylation and aromatization, which are associated with the carbonization of carbohydrates [16]. In the polymerization stage, as a result of intermolecular dehydration or aldol condensation, soluble polymers are formed [18]. The residence time affects the polymerization of degraded products in the liquid phase. A longer time increases the formation of microspheres and the polyaromatic hydrochar structure. When the pressure is too high, it can lead to a decrease in the intensity of decarboxylation and an increase in the intensity of polymerization [16][19].During the HTC of biomass, furfural is formed, and polymerization takes place, leading to the production of hydrochar. During the process, HTC decreases the oxygen and hydrogen content. Most of the carbon found in biomass remains in the produced hydrochar [20]. Its content depends on the feedstock and the condition of the process.
A significant aspect is energy recovery from the by-products of the HTC process. Both hydrochar and process water can be used for energy supply [21]. Process water contains various kinds of sugar, volatile fatty acids, and aromatic organic compounds. In addition, the dissolved organic carbon in this fraction accounts for up to 30% of the feedstock placed in the reactor. Water acts as a catalyst for the whole reaction and supports the conversion of organic waste into biofuel and organic liquid fraction. The severity of the process plays a key role in the carbon content of the final product. As it increased, the efficiency of the carbon content in hydrochar decreased. Hydrochar contains the largest amount of carbon of all three HTC products. The smallest amount of carbon is in the gas phase, the main component of which is carbon dioxide. This can cause a significant increase in reactor pressure above the water saturation pressure [20].
Maintaining the correct temperature and not too long a retention time [22]. For instance, Cao et al. [12] conducted the process at a temperature of 210 °C with different residence times: 30 min to 5 h. The digestate of cow manure and energy crops was the feedstock used in the HTC process, and half an hour of residence time resulted in a decrease in higher heating values. However, there was a boost in the slagging and fouling indexes observed. The 30 min process demonstrated the highest efficiency and better quality of the digestate [12].
Digestate is primarily used as fertilizer but can also be transformed into fuel pellets. The composition of digestate pellets depends on the organic waste used as feedstock in biogas plants. It is possible to use digestate as a combustion material, but it has a lower calorific value than pellets made of wood. The energy efficiency of digestate pellets is around 44 kW, and the competence of the process is 85%. For comparison, the combustion of wood pellets is higher than 90% [23][24].
As mentioned above, waste from agriculture and the food industry is mainly used in biogas production. Recently, research has been conducted on using algae as a substrate in the co-digestion process. The process occurs simultaneously, and the fermentation of a homogeneous mixture of different wastes occurs. As a result, more relevant parameters for the anaerobic digestion process are obtained [25].
Regardless of the selected parameters of the HTC process, the most important factor is the safety of the reactor operation. Due to changes in pressure and temperature, the reactor’s parameters should always be selected very carefully. In hydrothermal carbonization, different reactors can be used: batch, semi-batch and continuous, equipped with a stirrer, direct heating by steam injection or through the reactor’s walls. The cost of a high-pressure reactor can be relatively high. For that reason, most research is limited to studies concerning reasonable temperature and residence time ranges while considering the potential interest of investors. As a result of the formation of reaction gases and water vapour, the pressure is usually in the range of 0.6–6.4 MPa. In the HTC process, the raw material is immersed in liquid water, whereas when it has direct contact only with water vapour, it is called steam carbonization (VTC) [26].
Another type of HTC process is a “Two-stage HTC”, which consists of dividing the process into two phases. The first is hydrolysis, where products with a low molecular weight are obtained. It occurs at relatively low temperatures, up to 175 °C. The second phase occurs at higher temperatures, up to 280 °C, called carbonization. The hydrolysis products are dehydrated and polymerized. Consequently, “Two-stage HTC” reduces power consumption by up to 25% compared to the conventional HTC process. Besides, studies indicate that extending the hydrolysis time to 200 min increases the energy content of the produced hydrochar [27].
There is also a process called co-hydrothermal carbonization (co-HTC). This kind of process affects the properties of hydrochar. Studies were conducted for different materials, for example, sewage sludge with the addition of various types of organic waste, e.g., lignocellulosic, wood, food, agriculture, municipal solid waste, and sewage sludge. The latter kind of waste is characterized by its heterogeneity of structure and its fairly low carbon content. In addition, the high nitrogen and ash content reduces the efficiency of hydrochar as a solid fuel [28]. According to the data, wastewater treatment plants in Europe produce approximately 22.5 kg of dry matter of sludge per capita per year. It is estimated that annually this number reaches 17 million tonnes of dry sludge [29].
Furthermore, the HTC process has improved the drainage of the sewage sludge. Hydrochar, adding 20% fir enhanced the best properties of the obtained fuel [30]. Wilk et al. [31] also demonstrated that when the HTC process is applied to sewage sludge, it reduces the activation energy of hydrochars by 50% [31]. Liu et al. [32] used co-HTC for cellulose from wheat straw, xylan and soy protein and proved co-HTC has an impact on the efficiency of hydrochar. The highest hydrochar yield was observed for cellulose. In addition, co-HTC reduces the ratio of O:C and raises N:C in the resulting hydrochar. In addition, the effect of the co-HTC process with aqueous phase recirculation has been investigated [32]. Sample studies were conducted for aquatic plants of water hyacinth and cattail, as they have cellulose, hemicellulose, and lignin in their composition. According to previous studies, hydrochar obtained in the HTC process from those plants was characterized by better combustion efficiency and lower ash content [33].
Furthermore, the research confirmed the effectiveness of reducing the tested contaminants using these technologies [34]. Studies conducted for maize silage digestate as a substrate indicate that during the HTC process, increasing the temperature from 180 °C to 220 °C causes weight loss. The best parameters for the obtained hydrochar and process water were recorded at a temperature of 180 °C with a residence time of 30 min. Lower temperatures and shorter residence times cause a lack in the production of non-biodegradable or toxic substances in the process water [11].
In addition to the HTC process, two other processes stand out: hydrothermal liquefaction (HTL) and hydrothermal gasification (HTG). The first occurs within a temperature range of 200–400 °C and at a pressure of 10–25 MPa in an aqueous environment. As a result, the production of bio-petroleum occurs. In the HTG process, the conversion of biomass into gaseous components such as hydrogen, methane, and carbon dioxide. These gases can be used in heat production [15]. According to previous research, hydrochars contain greater values of higher calorific value and fixed carbon content than the initial feedstock. However, volatile matter and ash content are higher for agricultural waste [35].
This entry is adapted from the peer-reviewed paper 10.3390/en16145458
References
- Karlsson, N.P.E.; Halila, F.; Mattsson, M.; Hoveskog, M. Success Factors for Agricultural Biogas Production in Sweden: A Case Study of Business Model Innovation. J. Clean. Prod. 2017, 142, 2925–2934.
- Biomass. Available online: https://energy.ec.europa.eu/topics/renewable-energy/bioenergy/biomass_en (accessed on 5 February 2023).
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars Production, Characterization and Application for Wastewater Treatment: A Review. Renew. Sust. Energ. Rev. 2020, 127, 109882.
- Avila-Lopez, M.; Robles-Rodriguez, C.; Tiruta-Barna, L.; Ahmadi, A. Toward Thermal Autarky for Large-Scale Biogas Plants: Dynamic Energy Modeling for Energy Efficiency in Anaerobic Digesters with Enhanced Multimembrane Gasholders. Fuel 2022, 339, 126978.
- O’Connor, S.; Ehimen, E.; Pillai, S.C.; Black, A.; Tormey, D.; Bartlett, J. Biogas Production from Small-Scale Anaerobic Digestion Plants on European Farms. Renew. Sust. Energ. Rev. 2021, 139, 110580.
- Omar, B.; El-Gammal, M.; Abou-Shanab, R.; Fotidis, I.A.; Angelidaki, I.; Zhang, Y. Biogas Upgrading and Biochemical Production from Gas Fermentation: Impact of Microbial Community and Gas Composition. Bioresour. Technol. 2019, 286, 121413.
- Digestate Factsheet: The Value of Organic Fertilisers for Europe’s Economy, Society and Environment. Available online: http://europeanbiogas.eu/wp-content/uploads/2015/07/Digestate-paper-final-08072015.pdf (accessed on 20 January 2023).
- Skrzypczak, D.; Trzaska, K.; Mikula, K.; Gil, F.; Izydorczyk, G.; Mironiuk, M.; Polomska, X.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Conversion of Anaerobic Digestates from Biogas Plants: Laboratory Fertilizer Formulation, Scale-up and Demonstration of Applicative Properties on Plants. Renew. Energ. 2023, 203, 506–517.
- Hämäläinen, A.; Kokko, M.; Kinnunen, V.; Hilli, T.; Rintala, J. Hydrothermal Carbonisation of Mechanically Dewatered Digested Sewage Sludge—Energy and Nutrient Recovery in Centralised Biogas Plant. Water Res. 2021, 201, 117284.
- Jerzak, W.; Kuźnia, M.; Szajding, A. Experimental Studies and the Chemical Kinetics Modelling of Oxidation of Hydrogen Sulfide Contained in Biogas. Selected Papers from IX International Conference on Computational Heat and Mass Transfer (ICCHMT2016). Book Ser. Procedia Eng. 2016, 157, 222–229.
- Cao, Z.; Hülsemann, B.; Wüst, D.; Illi, L.; Oechsner, H.; Kruse, A. Valorization of Maize Silage Digestate from Two-Stage Anaerobic Digestion by Hydrothermal Carbonization. Energ. Convers. Manag. 2020, 222, 113218.
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of Residence Time during Hydrothermal Carbonization of Biogas Digestate on the Combustion Characteristics of Hydrochar and the Biogas Production of Process Water. Bioresour. Technol. 2021, 333, 125110.
- Śliz, M.; Tuci, F.; Czerwińska, K.; Fabrizi, S.; Lombardi, L.; Wilk, M. Hydrothermal Carbonization of the Wet Fraction from Mixed Municipal Solid Waste: Hydrochar Characteristics and Energy Balance. Waste Manag. 2022, 151, 39–48.
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal Carbonization Process: Fundamentals, Main Parameter Characteristics and Possible Applications Including an Effective Method of SARS-CoV-2 Mitigation in Sewage Sludge. A Review. Renew. Sust. Energ. Rev. 2022, 154, 111873.
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939.
- Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mecha-Nisms for Process Engineering. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/bbb.198?src=getftr (accessed on 28 February 2023).
- Leena, P.A.; Kurian, J. Hydrothermal Carbonization of Organic Wastes to Carbonaceous Solid Fuel—A Review of Mechanisms and Process Parameters. Fuel 2020, 279, 118472.
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon 2009, 47, 2281–2289.
- Lachos-Perez, D.; César Torres-Mayanga, P.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal Carbonization and Liquefaction: Differences, Progress, Challenges, and Opportunities. Bioresour. Technol. 2022, 343, 126084.
- Alvarez-Murillo, A.; Libra, J.A.; Ro, K.S. Theoretical Framework for Estimating Design Reactor Pressure for Water-Based Hydrothermal Carbonization (HTC) Systems. Therm. Sci. Eng. Prog. 2022, 30, 101241.
- Śliz, M.; Czerwińska, K.; Magdziarz, A.; Lombardi, L.; Wilk, M. Hydrothermal Carbonization of the Wet Fraction from Mixed Municipal Solid Waste: A Fuel and Structural Analysis of Hydrochars. Energies 2022, 15, 6708.
- Gaur, R.Z.; Khoury, O.; Zohar, M.; Poverenov, E.; Darzi, R.; Laor, Y.; Posmanik, R. Hydrothermal Carbonization of Sewage Sludge Coupled with Anaerobic Digestion: Integrated Approach for Sludge Management and Energy Recycling. Energy Convers. Manag. 2020, 224, 113353.
- Kratzeisen, M.; Starcevic, N.; Martinov, M.; Maurer, C.; Müller, J. Applicability of Biogas Digestate as Solid Fuel. Fuel 2010, 89, 2544–2548.
- Zaini, I.N.; Novianti, S.; Nurdiawati, A.; Irhamna, A.R.; Aziz, M.; Yoshikawa, K. Investigation of the Physical Characteristics of Washed Hydrochar Pellets Made from Empty Fruit Bunch. Fuel Process. Technol. 2017, 160, 109–120.
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A Critical Review on Anaerobic Digestion of Microalgae and Macroalgae and Co-Digestion of Biomass for Enhanced Methane Generation. Bioresour. Technol. 2018, 262, 319–332.
- Ro, K.S.; Libra, J.A.; Alvarez-Murillo, A. Comparative Studies on Water-and Vapor-Based Hydrothermal Carbonization: Process Analysis. Energies 2020, 13, 5733.
- Fakkaew, K.; Koottatep, T.; Polprasert, C. Effects of Hydrolysis and Carbonization Reactions on Hydrochar Production. Bioresour. Technol. 2015, 192, 328–334.
- Djandja, O.S.; Kang, S.; Huang, Z.; Li, J.; Feng, J.; Tan, Z.; Salami, A.A.; Lougou, B.G. Machine Learning Prediction of Fuel Properties of Hydrochar from Co-Hydrothermal Carbonization of Sewage Sludge and Lignocellulosic Biomass. Energy 2023, 271, 126968.
- The Changing Landscape of Sludge Disposal. Available online: https://www.solenis.com/en/resources/blog/the-changing-landscape-of-sludge-disposal#:~:text=Worldwide%20sludge%20production%20is%20extensive%20and%20growing.%20In,17%20million%20tons%20of%20dry%20sludge%20per%20year (accessed on 20 March 2023).
- Wilk, M.; Śliz, M.; Lubieniecki, B. Hydrothermal Co-Carbonization of Sewage Sludge and Fuel Additives: Combustion Performance of Hydrochar. Renew. Energy 2021, 178, 1046–1056.
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal Carbonization Characteristics of Sewage Sludge and Lignocellulosic Biomass. A Comparative Study. Biomass. Bioenergy 2019, 120, 166–175.
- Liu, T.; Jiao, H.T.; Yang, L.; Zhang, W.; Hu, Y.; Guo, Y.; Yang, L.; Leng, S.; Chen, J.; Chen, J.; et al. Co-Hydrothermal Carbonization of Cellulose, Hemicellulose, and Protein with Aqueous Phase Recirculation: Insight into the Reaction Mechanisms on Hydrochar Formation. Energy 2022, 251, 123965.
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal conversion of different lignocellulosic biomass feedstocks—Effect of the process conditions on hydrochar structures. Fuel 2021, 302, 121166.
- Czerwińska, K.; Marszałek, A.; Kudlek, E.; Śliz, M.; Dudziak, M.; Wilk, M. The Treatment of Post-Processing Liquid from the Hydrothermal Carbonization of Sewage Sludge. Sci. Total Environ. 2023, 885, 163858.
- Wang, S.; Wen, Y.; Shi, Z.; Niedzwiecki, L.; Baranowski, M.; Czerep, M.; Mu, W.; Kruczek, H.P.; Jönsson, P.G.; Yang, W. Effect of Hydrothermal Carbonization Pretreatment on the Pyrolysis Behavior of the Digestate of Agricultural Waste: A View on Kinetics and Thermodynamics. Chem. Eng. J. 2022, 431, 133881.
This entry is offline, you can click here to edit this entry!
