Antibiotics are among the most important discoveries of the 20th century, having saved millions of lives from infectious diseases. Microbes have developed acquired antimicrobial resistance (AMR) to many drugs due to high selection pressure from increasing use and misuse of antibiotics over the years. The transmission and acquisition of AMR occur primarily via a human–human interface both within and outside of healthcare facilities. A huge number of interdependent factors related to healthcare and agriculture govern the development of AMR through various drug-resistance mechanisms. The emergence and spread of AMR from the unrestricted use of antimicrobials in livestock feed has been a major contributing factor. The prevalence of antimicrobial-resistant bacteria has attained an incongruous level worldwide and threatens global public health as a silent pandemic, necessitating urgent intervention.
- antibiotics
- antimicrobial resistance
- mechanisms of resistance
- drivers of resistance
- measures to combat resistance
1. Introduction
2. Superbugs
3. Basis of Antibiotic Resistance
4. Sources and Routes of Transmission of AMR
5. Mechanisms of Drug Resistance
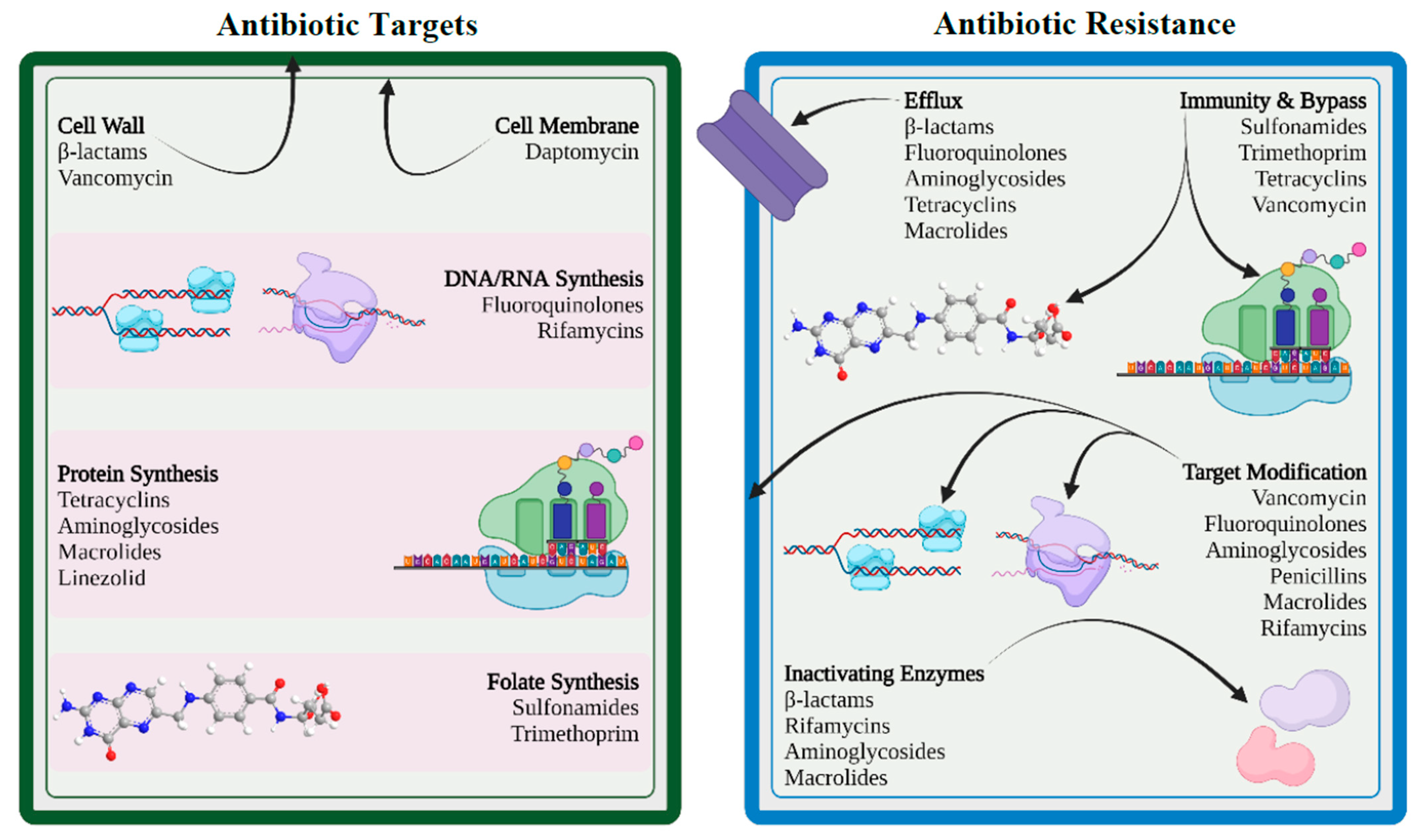
5.1. Limiting Drug Uptake
5.2. Modification of Targets for Drug
5.3. Inactivation of Drug
5.4. Efflux of Drug
6. Drivers to AMR
6.1. Misuse and Overuse of Antibiotics
6.2. Increase in Gross Domestic Product (GDP)
6.3. Inappropriate Prescribing Patterns
6.4. Paucity in Futuristic Antibiotics
6.5. Agricultural Use of Antibiotics
6.6. Easy Travel Routes
6.7. Knowledge Gap
7. Clinical Implications of AMR
-
Successful treatment of microbial infections including bacterial, fungal, and viral infections is hindered by antimicrobial resistance.
-
Emergence and dissemination of new resistant mechanisms threaten the scope of treatment for many common illnesses such as urinary tract infections, upper respiratory tract infections, typhoid, and flu, resulting in treatment failure, permanent disability, or even death.
-
The success of cancer chemotherapy, transplantation surgery, and even minor dental procedures will be seriously jeopardized by virtue of AMR unless novel drugs are available.
-
AMR infections impose mandatory prolonged treatment with higher healthcare costs and may require expensive alternate drugs.
8. How to Combat AMR
8.1. International Measures
-
Establishing and strengthening collaboration among international agencies, governments, nongovernmental organizations, and professional groups.
-
Establishing surveillance networks for antimicrobial use and AMR globally.
-
Building laboratory capacity for the detection and reporting of pathogens with AMR that have global health impacts.
-
Establishing and strengthening international tracking systems for quick identification and mitigation of emerging pathogens.
-
International monitoring to control counterfeit antimicrobials across the globe.
-
Investing in research, new drug discovery, and vaccines.
8.2. National Strategies
-
Implementing an “Antibiotic policy” for judicious use in healthcare and agricultural settings.
-
Strengthening of national surveillance, monitoring, and evaluation efforts by integration of public health and veterinary sectors.
-
Developing innovative point-of-care diagnostic tests for pathogen identification and resistance monitoring.
-
Investing in basic and applied research on new antibiotics and vaccines.
-
Building capacity and strengthening international collaboration to combat AMR.
-
Adopting antimicrobial stewardship in healthcare settings with essential drug list.
8.3. Rational Use of Antibiotics
8.4. Ban on Over-the-Counter (OTC) Antibiotics
8.5. Infection Prevention and Control (IPC)
-
Formation of “infection prevention and control committee”.
-
Practices of good hand hygiene.
-
Proper diagnosis and successful treatment of infection.
-
Responsible use of antimicrobial agents.
-
Continuous surveillance and monitoring of antibiotic use and antibiotic resistance.
-
Establishing quality antimicrobials supply chain.
-
Good microbiological laboratory practices.
8.6. Antimicrobial Stewardship Program (ASP)
8.7. Use of Antibiotics in Animals
8.8. Development of New Drugs and Vaccines
8.9. Introduction of Checkpoints
8.10. Community Engagement
8.11. Alternatives to Antibiotics
This entry is adapted from the peer-reviewed paper 10.3390/healthcare11131946
References
- Williams-Nguyen, J.; Sallach, J.B.; Bartelt-Hunt, S.; Boxall, A.B.; Durso, L.M.; McLain, J.E.; Singer, R.S.; Snow, D.D.; Zilles, J.L. Antibiotics and Antibiotic Resistance in Agroecosystems: State of the Science. J. Environ. Qual. 2016, 45, 394–406.
- CDC. How Antimicrobial Resistance Happens. Available online: https://www.cdc.gov/drugresistance/about/how-resistance-happens.html (accessed on 2 June 2023).
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10; discussion S62–S70.
- Zhou, G.; Shi, Q.S.; Huang, X.M.; Xie, X.B. The Three Bacterial Lines of Defense against Antimicrobial Agents. Int. J. Mol. Sci. 2015, 16, 21711–21733.
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42.
- Read, A.F.; Woods, R.J. Antibiotic resistance management. Evol. Med. Public Health 2014, 2014, 147.
- George, A. Antimicrobial resistance, trade, food safety and security. One Health 2018, 5, 6–8.
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111.
- ARC. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655.
- Kaur, N.; Prasad, R.; Varma, A. Prevalence and antibiotic susceptibility pattern of methicillin resistant staphylococcus aureus in tertiary care hospitals. Biotechnol. J. Int. 2014, 4, 228–235.
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388.
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 417–433.
- Lee, J.H. Perspectives towards antibiotic resistance: From molecules to population. J. Microbiol. 2019, 57, 181–184.
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today. Technol. 2014, 11, 33–39.
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. IJMM 2013, 303, 287–292.
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187.
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4.
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681.
- Rizi, K.S.; Ghazvini, K.; Noghondar, M.K. Adaptive antibiotic resistance: Overview and perspectives. J. Infect. Dis. Ther. 2018, 6, 363.
- Godijk, N.G.; Bootsma, M.C.J.; Bonten, M.J.M. Transmission routes of antibiotic resistant bacteria: A systematic review. BMC Infect. Dis. 2022, 22, 482.
- Krzemiński, P.; Markiewicz, Z.; Popowska, M. Entry Routes of Antibiotics and Antimicrobial Resistance in the Environment. In Antibiotics and Antimicrobial Resistance Genes: Environmental Occurrence and Treatment Technologies; Hashmi, M.Z., Ed.; Springer International Publishing: Cham, Germany, 2020; pp. 1–26.
- da Costa, P.M.; Loureiro, L.; Matos, A.J. Transfer of multidrug-resistant bacteria between intermingled ecological niches: The interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294.
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22.
- Choi, U.; Lee, C.R. Distinct Roles of Outer Membrane Porins in Antibiotic Resistance and Membrane Integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953.
- Ghai, I.; Ghai, S. Understanding antibiotic resistance via outer membrane permeability. Infect. Drug Resist. 2018, 11, 523–530.
- Ashley, R.E.; Dittmore, A.; McPherson, S.A.; Turnbough, C.L., Jr.; Neuman, K.C.; Osheroff, N. Activities of gyrase and topoisomerase IV on positively supercoiled DNA. Nucleic Acids Res. 2017, 45, 9611–9624.
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363.
- Chaw, P.S.; Höpner, J.; Mikolajczyk, R. The knowledge, attitude and practice of health practitioners towards antibiotic prescribing and resistance in developing countries-A systematic review. J. Clin. Pharm. Ther. 2018, 43, 606–613.
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42.
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654.
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145.
- Pulia, M.; Kern, M.; Schwei, R.J.; Shah, M.N.; Sampene, E.; Crnich, C.J. Comparing appropriateness of antibiotics for nursing home residents by setting of prescription initiation: A cross-sectional analysis. Antimicrob. Resist. Infect. Control 2018, 7, 74.
- Woolhouse, M.; Waugh, C.; Perry, M.R.; Nair, H. Global disease burden due to antibiotic resistance-state of the evidence. J. Glob. Health 2016, 6, 010306.
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33.
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247.
- FAO. Animal production. Available online: https://www.fao.org/antimicrobial-resistance/key-sectors/animal-production/en/ (accessed on 12 November 2022).
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.J.; van Genderen, P.J.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): A prospective, multicentre cohort study. Lancet. Infect. Dis. 2017, 17, 78–85.
- McCubbin, K.D.; Anholt, R.M.; de Jong, E.; Ida, J.A.; Nóbrega, D.B.; Kastelic, J.P.; Conly, J.M.; Götte, M.; McAllister, T.A.; Orsel, K.; et al. Knowledge Gaps in the Understanding of Antimicrobial Resistance in Canada. Front. Public Health 2021, 9, 726484.
- Carter, R.R.; Sun, J.; Jump, R.L. A Survey and Analysis of the American Public's Perceptions and Knowledge About Antibiotic Resistance. Open Forum Infect. Dis. 2016, 3, ofw112.
- WHO. Antibiotic resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 12 November 2022).
- Lin, T.Z.; Jayasvasti, I.; Tiraphat, S.; Pengpid, S.; Jayasvasti, M.; Borriharn, P. The Predictors Influencing the Rational Use of Antibiotics Among Public Sector: A Community-Based Survey in Thailand. Drug Healthc. Patient Saf. 2022, 14, 27–36.
- WHO. Assessing Non-Prescription and Inappropriate Use of Antibiotics Report on Survey. Available online: https://apps.who.int/iris/bitstream/handle/10665/312306/9789289054089-eng.pdf?sequence=1&isAllowed=y (accessed on 4 June 2023).
- CDC. Implementation of Antibiotic Stewardship Core Elements at Small and Critical Access Hospitals. Available online: https://www.cdc.gov/antibiotic-use/core-elements/small-critical.html (accessed on 12 November 2022).
- Pinto Ferreira, J.; Battaglia, D.; Dorado García, A.; Tempelman, K.; Bullon, C.; Motriuc, N.; Caudell, M.; Cahill, S.; Song, J.; LeJeune, J. Achieving Antimicrobial Stewardship on the Global Scale: Challenges and Opportunities. Microorganisms 2022, 10, 1599.
- Aidara-Kane, A.; Angulo, F.J.; Conly, J.M.; Minato, Y.; Silbergeld, E.K.; McEwen, S.A.; Collignon, P.J. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 2018, 7, 7.
- WHO. Global Action Plan on Antibiotic Resistance. Available online: https://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html (accessed on 12 November 2022).
- WHO. Infection Prevention and Control. Available online: https://www.who.int/teams/integrated-health-services/infection-prevention-control (accessed on 12 November 2022).
- Mitchell, J.; Cooke, P.; Ahorlu, C.; Arjyal, A.; Baral, S.; Carter, L.; Dasgupta, R.; Fieroze, F.; Fonseca-Braga, M.; Huque, R.; et al. Community engagement: The key to tackling Antimicrobial Resistance (AMR) across a One Health context? Glob. Public Health 2022, 17, 2647–2664.
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911.
- Al-Amin, M.Y.; Lahiry, A.; Ferdous, R.; Hasan, M.K.; Kader, M.A.; Alam, A.K.; Saud, Z.A.; Sadik, M.G. Stephania japonica Ameliorates Scopolamine-Induced Memory Impairment in Mice through Inhibition of Acetylcholinesterase and Oxidative Stress. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 8305271.
- Foyzun, T.; Mahmud, A.A.; Ahammed, M.S.; Manik, M.I.N.; Hasan, M.K.; Islam, K.M.M.; Lopa, S.S.; Al-Amin, M.Y.; Biswas, K.; Afrin, M.R.; et al. Polyphenolics with Strong Antioxidant Activity from Acacia nilotica Ameliorate Some Biochemical Signs of Arsenic-Induced Neurotoxicity and Oxidative Stress in Mice. Molecules 2022, 27, 1037.
- Islam, M.A.; Zaman, S.; Biswas, K.; Al-Amin, M.Y.; Hasan, M.K.; Alam, A.; Tanaka, T.; Sadik, G. Evaluation of cholinesterase inhibitory and antioxidant activity of Wedelia chinensis and isolation of apigenin as an active compound. BMC Complement. Med. Ther. 2021, 21, 204.
- Mustafa, S.; Akbar, M.; Khan, M.A.; Sunita, K.; Parveen, S.; Pawar, J.S.; Massey, S.; Agarwal, N.R.; Husain, S.A. Plant metabolite diosmin as the therapeutic agent in human diseases. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100122.
- Pawar, J.S.; Mustafa, S.; Ghosh, I. Chrysin and Capsaicin induces premature senescence and apoptosis via mitochondrial dysfunction and p53 elevation in Cervical cancer cells. Saudi J. Biol. Sci. 2022, 29, 3838–3847.
- Kundo, N.K.; Manik, M.I.N.; Biswas, K.; Khatun, R.; Al-Amin, M.Y.; Alam, A.; Tanaka, T.; Sadik, G. Identification of Polyphenolics from Loranthus globosus as Potential Inhibitors of Cholinesterase and Oxidative Stress for Alzheimer's Disease Treatment. BioMed. Res. Int. 2021, 2021, 9154406.
- Amaning Danquah, C.; Minkah, P.A.B.; Osei Duah Junior, I.; Amankwah, K.B.; Somuah, S.O. Antimicrobial Compounds from Microorganisms. Antibiotics 2022, 11, 285.
