Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Due to low sludge production and being a clean source without residuals, hydrogen-based autotrophic denitrification appears to be a promising choice for nitrate removal from agricultural drainage waters or water/wastewater with a similar composition. Although the incorporation of hydrogen-based autotrophic denitrification with membrane bioreactors (MBRs) enabled almost 100% utilization of hydrogen, the technology still needs to be improved to better utilize its advantages.
- anoxic denitrification
- membrane bioreactor (MBR)
- fouling
- venturi
- hydrogen
1. Environmental Effects of Agricultural Drainage Waters
In agricultural areas where natural drainage is insufficient, the wastewater formed due to the removal of unused irrigation water from the land with drainage channels is called agricultural drainage water [1]. Agricultural drainage waters containing salt, trace elements and other pollutants can damage both soil and aquatic ecosystems when discharged into receiving water environments. Excessive nitrogen that is not used by plants and animals in agricultural systems can percolate into shallow groundwater and eventually enter surface waters through concentrated or diffuse discharges [2]. For example, 25% of 248 groundwater well samples taken from a large area in Mexico, which meets 10% of citrus production, exceeded the national drinking water standard of 10 mg/L NO3−–N concentration [3].
Although many studies have been conducted on the reuse of domestic and industrial wastewater, little attention has been paid to the reuse of agricultural drainage waters after proper treatment. Drainage waters are reused in various parts of the world without treatment, but this may cause problems such as soil salinization, decreased permeability, rise in groundwater level, etc.
Since approximately 3/4 of the clean water used contributes to agricultural irrigation, these waters have great recovery potential. This rate is 70% worldwide [4], and 77% in Turkey [5]. For instance, in the measurements made by the 15th Regional Directorate of State Water Works (DSI), the amount of water removed by the main drainage channel in the Harran Plain (Sanliurfa, Turkey) has been reported to be 190–200 million m3/year in recent years [6]. This amount is considerably higher than the annual drinking water need of Sanliurfa, which had a population of 1,985,000 in 2017.
2. Treatment Methods for Agricultural Drainage Waters
Treatment of agricultural drainage waters is the last resort option in drainage water management, due to the high cost of treatment and the uncertainty of achieving the desired level of treatment [7]. One of the distinctive features of agricultural drainage waters is the insufficient carbon source required for biological treatment. Zhang et al. [8] investigated the ability of five different bacteria to use molasses as a carbon source in their study, in which they used molasses (sugar residue) as a carbon source to reduce selenate (Se+6) in the drainage water to elemental selenium (Se0) using bacteria. The study found that Enterobacter taylorae converts selenate to selenium with an efficiency of 97% in artificial drainage water containing 1000 μg/L selenate, and that molasses could be used as an economical carbon source in drainage water treatment.
Quinn et al. [9] established a demonstration plant with algal–bacterial treatment to remove nitrate and selenium from drainage water. The process achieved over 95% nitrate removal and 80% dissolved selenium removal. Hunt et al. [10] investigated the denitrification efficiency of biomass that adheres to polyvinyl alcohol (PVA) material in agricultural drainage water. The study reported a 50% removal efficiency during a 1 h soaking period in water containing 7.8 mg 𝑁𝑂−3–𝑁O3−–N/L. The nitrate removal rate was also high, with a value of 94 g 𝑁𝑂−3–N/m2/day.
Allred [11] investigated the removal of nitrate and phosphate from agricultural drainage waters through filtration using filter materials formed by reacting iron particles with zero-valent iron and sulfur. The intermittent experiments showed that both filter materials achieved at least 94% phosphate removal and 86–88% nitrate removal. Messer et al. [12] conducted a study utilizing two distinct wetland restoration sites with different soils (organic and mineral soils) for treating 𝑁O3−–N-rich synthetic agricultural drainage water. The results showed that the mineral soil wetland achieved a better performance than organic soil in terms of nitrate removal, which was attributed to higher plant uptake in the mineral soil due to its limited nitrogen content. In another study, Lavrnic et al. [12] examined the long-term performance of a full-scale surface flow-constructed wetland in terms of nitrogen removal rates, among other parameters. Two-year monitoring showed up to 78% removal of 𝑁O3−–N.
3. Membrane Bioreactors
According to Judd [13], all processes that combine a selective membrane with biological water and wastewater treatment to capture solids are called membrane bioreactors (MBRs). The use of MBRs in water and wastewater treatment has some advantages, such as high biomass concentrations and high organic loading rates due to high sludge ages, better output quality in terms of turbidity, bacteria, particulate and colloidal organic matter, and a reduction in treatment cost of industrial sludges due to lower sludge production [14][15]. However, membrane fouling and the associated high operating cost can be considered the main disadvantages of MBRs.
Membrane fouling is the clogging of the membrane pores due to foulants accumulating on the membrane, leading to a reduction in the membrane flux and an increase in transmembrane pressure. When the membrane fouling reaches an advanced level, the membrane cannot be used further, and it must be cleaned physically and chemically. Membrane fouling can have many reasons depending on the inlet wastewater characteristics, the membrane and module itself, and operating conditions.
The most widely used method to reduce membrane fouling is to scour the membrane surface with a gas [16]. This is valid for both aerobic and anaerobic membrane bioreactors [17]. While air is mostly used in aerobic reactors (e.g., [18]), headspace gas is generally used in anaerobic conditions [19].
4. Hydrogenotrophic Denitrification
In the removal of nitrates from agricultural drainage waters by heterotrophic denitrification, an external organic carbon source needs to be added. Several organic carbon sources such as methanol [10], acetate, lactate, and glucose [20] were used in previous studies. However, some of this externally added organic carbon is utilized by selenate-reducing bacteria, and most of it is removed with the effluent stream, causing an increase in the cost of treatment due to the wasted expensive chemicals [20]. As an alternative economic source of organic carbon, Zhang et al. [21] investigated rice straw, and Zhang et al. [8] studied the usability of molasses.
In agricultural drainage waters with an insufficient carbon content, nitrate removal using the autotrophic denitrification process, which does not require an organic carbon source, has significant potential. The autotrophic denitrification processes can be divided into two basic groups based on the electron donor: hydrogen-based and sulfur-based reactions. However, the requirement for limestone to produce sulfates and for pH adjustment severely limits the applicability of sulfur-based denitrification. Hydrogen-based denitrification by species such as Ochrobactrum anthropi, Pseudomonas strutzeri, Paracoccus denitrificans and Paracoccus panthotrophus has two main advantages over sulfur-using denitrification and heterotrophic denitrification. One advantage of hydrogen-based denitrification is that it is practically impossible for the given electron donor to be left behind, since when water (or wastewater) is exposed to the open surface, H2 is released into the atmosphere. Additionally, H2 does not leave any by-products that may affect the effluent quality [22]. Another advantage of hydrogenotrophic denitrification is that it does not require post-treatment [23] and has low sludge production. Hence, the applicability of the hydrogenotrophic denitrification process has been investigated for the removal of various pollutants such as perchlorate, selenate, chromate, arsenate, trichloroethene, trichloroethane and chloroform [24].
Lee and Rittmann [25] achieved 76% nitrate removal efficiency under 0.31 atm H2 pressure in synthetic wastewater containing 10 mg/L 𝑁O3−–N. When the inlet 𝑁O3−–N concentration was increased to 12.5 mg/L and H2 pressure to 0.42 atm, 92% nitrate removal efficiency was obtained. Visvanathan et al. (2008) stated that hydraulic retention times of 3, 5 and 6 h, respectively, were necessary to achieve a removal efficiency of over 90% in synthetic wastewater containing 50 mg/L 𝑁O3−–N and 1%, 2% and 3% NaCl. During these hydraulic retention times, the denitrification rates of the system were 366.8, 226.2 and 193.2 g/m3/day.
The stoichiometry of the hydrogenotrophic denitrification process is as follows [23]:
According to Equation (1), 1 mg of 𝑁O3−–N requires 0.357 mg of H2 gas. Also, a reduction of 1 meq of 𝑁O3−–N produces 1 meq of alkalinity (equivalent to 3.57 mg CaCO3).
Denitrification with hydrogen has two notable drawbacks. Firstly, hydrogen gas is not highly soluble in water. Secondly, it can be explosive in the air within the range of 4–75% [25]. The most common approach is to introduce hydrogen gas into the reactor either with a tank that is saturated with hydrogen or directly into the reactor. However, the mass transfer rate is low due to its low solubility, and there is a risk of explosion if it accumulates in a sealed place [23]. To overcome these disadvantages, membrane biofilm reactors have been developed. These reactors use gas transfer membranes to supply hydrogen, preventing the formation of bubbles. In these reactors, it has been reported that hydrogen is used with up to 100% efficiency [25].
However, membrane biofilm reactors also have some issues. In their study, Lee and Rittmann [25] found that the biofilm that developed on hollow fiber membranes was non-uniform. They found that the biofilm was thicker in the areas closest to the gas supply. The growth of biofilm on the membrane can decrease the rate of gas transfer. This can cause difficulties when there is leakage of gas from fibers or fiber regions with a thinner biofilm [22]. In this configuration, hydrogen and nitrate are supplied to opposite sides of a membrane. However, there is a challenge in that they diffuse in opposite directions, which makes the process more difficult. This means that the highest concentrations of hydrogen and nitrate are not typically found in the same place. This may limit the transfer of both substances [22]. Similarly, Visvanathan et al. [26] reported an increase of 16–31% in resistance due to fouling of the membrane used for gas transfer. However, its efficiency was restored after cleaning with chemicals.
A new MBR configuration has been developed that increases the solubility of hydrogen gas in the MBR and reduces membrane fouling [27]. In this configuration, the activated sludge suspension is circulated through a venturi injector with the headspace gas and then returned to the reactor. The cavitation in the venturi increases gas transfer, while a diffuser directs the liquid–gas mixture to the membrane surface for better scouring. However, the effect of headspace gas circulation or the means of circulation, whether by a venturi device or peristaltic pump, on membrane fouling and denitrification process performance was not studied, although MBR configuration was partly the same.
5. Venturi Injectors
A venturi tube consists of three parts: the conical narrowing region, the throat, and the conical expansion region (Figure 1). In the contraction zone, there is a decrease in pressure parallel to the increase in velocity. However, the velocity is converted back to pressure with some loss in the expansion zone.
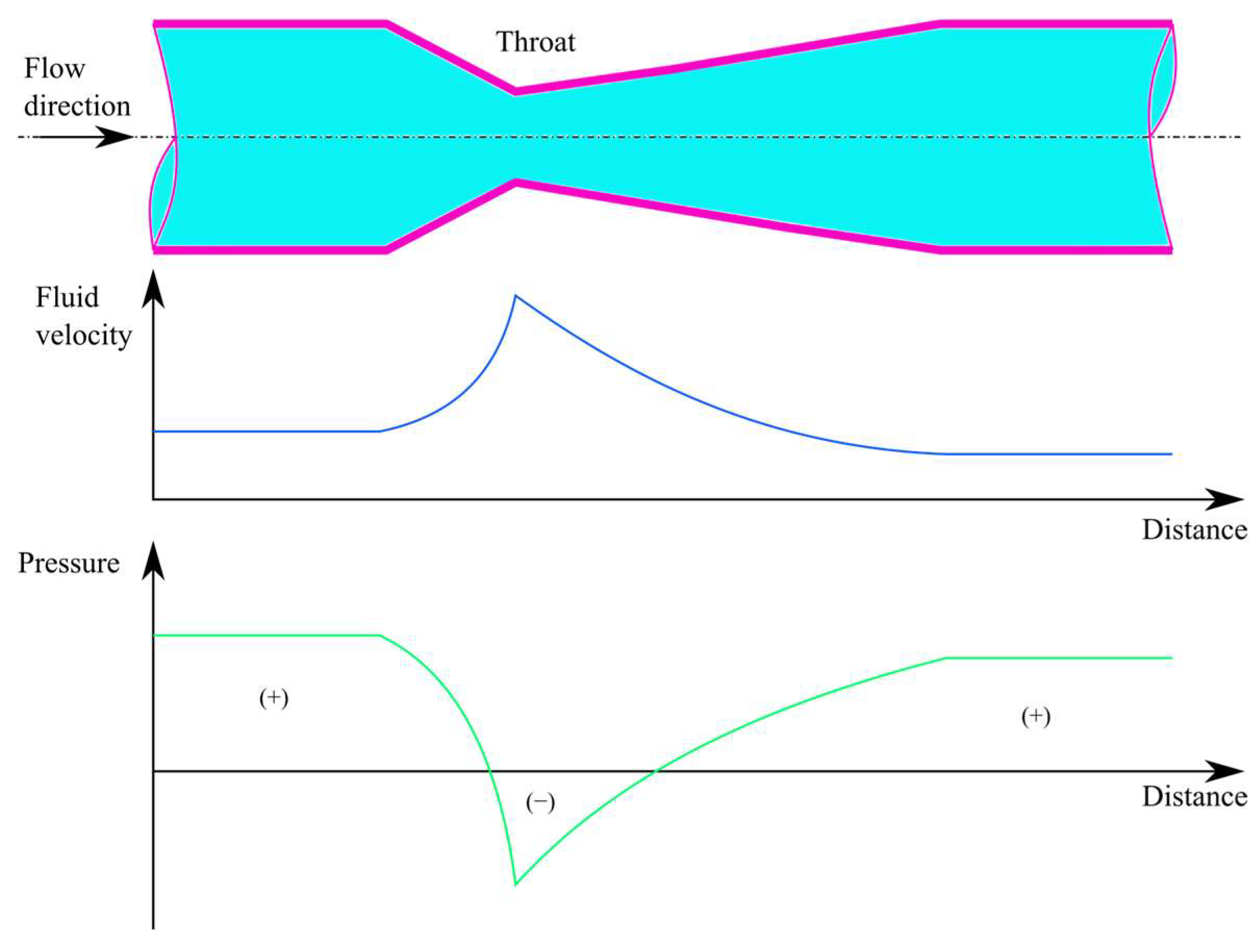
Figure 1. The change in fluid velocity and pressure along the flow direction in a venturi device.
Venturi injectors are utilized in various applications due to their ability to increase mass transfer through hydrodynamic cavitation occurring in the throat part of the device.
According to Englehart et al. [28], a typical bubble diffuser system has an O3 mass transfer efficiency of around 10–15%, while a system using a venturi injector can achieve a transfer efficiency of 90%. Venturi injectors have been used in full-scale wastewater treatment plants to increase oxygen transfer efficiency (e.g., [29]).
This entry is adapted from the peer-reviewed paper 10.3390/membranes13070666
References
- Causape, J.; Quilez, D.; Aragües, R. Salt and nitrate concentrations in the surface waters of the CR-V irrigation district (Bardenas I, Spain): Diagnosis and prescriptions for reducing off-site contamination. J. Hydrol. 2004, 295, 87–100.
- Mastrocicco, M.; Colombani, N.; Giuseppe, D.D.; Faccini, B.; Coltorti, M. Contribution of the subsurface drainage system in changing the nitrogen speciation of an agricultural soil located in a complex marsh environment (Ferrara, Italy). Agric. Water Manag. 2013, 119, 144–153.
- Pasten-Zapata, E.; Ledesma-Ruiz, R.; Harter, T.; Ramirez, A.I.; Mahlknecht, J. Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci. Total Environ. 2014, 470–471, 855–864.
- FAO. Water Uses. Available online: https://www.fao.org/aquastat/en/overview/methodology/water-use (accessed on 7 December 2022).
- DSI (State Water Works) Soil Water Resources. Available online: https://www.dsi.gov.tr/Sayfa/Detay/754 (accessed on 7 December 2022).
- Yurtseven, E.; Cakmak, B.; Kesmez, D.; Polat, H.E. Reuse of Agricultural Drainage Waters in Irrigation. In Proceedings of the Turkish Agricultural Engineering 7th Congress, Ankara, Turkey, 11 January 2010.
- Tanji, K.K.; Kielen, N.C. Agricultural Drainage Water Management in Arid and Semi-Arid Areas; Food And Agriculture Organization of The United Nations: Rome, Italy, 2002; Volume Paper 61, ISBN 9251048398.
- Zhang, Y.; Okeke, B.C.; Frankenberger, W.T., Jr. Bacterial reduction of selenate to elemental selenium utilizing molasses as a carbon source. Bioresour. Technol. 2008, 99, 1267–1273.
- Quinn, N.W.T.; Leighton, T.; Lundquist, T.J.; Green, F.B.; Zárate, M.A.; Oswald, W.J. Algal-Bacterial Treatment Facility Removes Selenium from Drainage Water. Calif. Agric. 2000, 54, 50–56.
- Hunt, P.G.; Matheny, T.A.; Ro, K.S.; Stone, K.C.; Vanotti, M.B. Denitrification of Agricultural Drainage Line Water via Immobilized Denitrification Sludge. J. Environ. Sci. Health Part A 2008, 43, 1077–1084.
- Allred, B.J. Laboratory Evaluation of Zero Valent Iron and Sulfur-Modified Iron for Agricultural Drainage Water Treatment. Groundwater Monit. Remediat. 2012, 32, 81–95.
- Messer, T.L.; Burchell, M.R.; Birgand, F.; Broome, S.W.; Chescheir, G. Nitrate Removal Potential of Restored Wetlands Loaded with Agricultural Drainage Water: A Mesocosm Scale Experimental Approach. Ecol. Eng. 2017, 106, 541–554.
- Judd, S. The MBR Book, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011.
- Pollice, A.; Laera, G.; Saturno, D.; Giordano, C. Effects of Sludge Retention Time on the Performance of a Membrane Bioreactor Treating Municipal Sewage. J. Memb. Sci. 2008, 317, 65–70.
- Casey, E. Membrane Bioreactors for Wastewater Treatment. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; Pabby, A.K., Rizvi, S.S.H., Sastre, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1007–1008.
- Vinardell, S.; Sanchez, L.; Astals, S.; Mata-Alvarez, J.; Dosta, J.; Heran, M.; Lesage, G. Impact of Permeate Flux and Gas Sparging Rate on Membrane Performance and Process Economics of Granular Anaerobic Membrane Bioreactors. Sci. Total Environ. 2022, 825, 153907.
- Wang, K.M.; Cingolani, D.; Eusebi, A.L.; Soares, A.; Jefferson, B.; McAdam, E.J. Identification of Gas Sparging Regimes for Granular Anaerobic Membrane Bioreactor to Enable Energy Neutral Municipal Wastewater Treatment. J. Memb. Sci. 2018, 555, 125–133.
- Meng, F.; Yang, F.; Shi, B.; Zhang, H. A Comprehensive Study on Membrane Fouling in Submerged Membrane Bioreactors Operated under Different Aeration Intensities. Sep. Purif. Technol. 2008, 59, 91–100.
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic Membrane Bioreactors for Wastewater Treatment: Novel Configurations, Fouling Control and Energy Considerations. Bioresour. Technol. 2019, 283, 358–372.
- Zhang, Y.; Frankenberger, W.T. Removal of Selenium from River Water by a Microbial Community Enhanced with Enterobacter Taylorae in Organic Carbon Coated Sand Columns. Sci. Total Environ. 2005, 346, 280–285.
- Zhang, Y.; Frankenberger, W.T. Factors Affecting Removal of Selenate in Agricultural Drainage Water Utilizing Rice Straw. Sci. Total Environ. 2003, 305, 207–216.
- Celmer-Repin, D.; Hwang, J.H.; Cicek, N.; Oleszkiewicz, J.A. Autotrophic Nitrogen-Removing Biofilms on Porous and Non-Porous Membranes. Environ. Technol. 2010, 31, 1391–1401.
- Karanasios, K.A.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Hydrogenotrophic Denitrification of Potable Water: A Review. J. Hazard Mater. 2010, 180, 20–37.
- Hasar, H. Simultaneous Removal of Organic Matter and Nitrogen Compounds by Combining a Membrane Bioreactor and a Membrane Biofilm Reactor. Bioresour. Technol. 2009, 100, 2699–2705.
- Lee, K.C.; Rittmann, B.E. A Novel Hollow-Fibre Membrane Biofilm Reactor for Autohydrogenotrophic Denitrification of Drinking Water. Water Sci. Technol. 2000, 41, 219–226.
- Visvanathan, C.; Phong, D.D.; Jegatheesan, V. Hydrogenotrophic Denitrification of Highly Saline Aquaculture Wastewater Using Hollow Fiber Membrane Bioreactor. Environ. Technol. 2008, 29, 701–707.
- Gül, E.; Kayaalp, N. Modelling of Hydrogenotrophic Denitrification Process in a Venturi-Integrated Membrane Bioreactor. Env. Technol. 2022, 1–14.
- Englehardt, J.D.; Wu, T.; Tchobanoglous, G. Urban Net-Zero Water Treatment and Mineralization: Experiments, Modeling and Design. Water Res. 2013, 47, 4680–4691.
- Mazzei Injector Company Wastewater Airjection Systems. Available online: http://mazzei.net/files/MAZ_WW_AirjectionProductSheet.pdf (accessed on 7 December 2022).
This entry is offline, you can click here to edit this entry!
