Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Dendrimers, being highly branched monodispersed macromolecules, predominantly exhibit identical terminal functionalities within their structural framework. Nonetheless, there are instances where the presence of two distinct surface functionalities becomes advantageous for the fulfilment of specific properties. To achieve this objective, one approach involves implementing Janus dendrimers, consisting of two dendrimeric wedges terminated by dissimilar functionalities.
- phosphorus chemistry
- Janus dendrimers
- dendrons
- 31P NMR spectroscopy
1. Introduction
Dendrimers are highly branched macromolecules characterized in most cases by a spherical shape. This unique class of polymers is often constructed through step-by-step reactions, starting from a polyfunctional core and progressively growing over generations. At each generation, new branching points are introduced, leading to an exponential increase in the number of terminal functional groups [1][2]. Dendrimers have garnered significant attention in the scientific literature and are considered versatile systems with a wide range of applications in various fields such as biology [3], biomedicine [1][4], material science [5][6], catalysis [7][8][9], or sensors [10][11], among others. However, the functionalization of dendrimer surfaces has been predominantly limited to a uniformity of functional groups, restricting their applicability to a single specific area.
To overcome this limitation and enable the incorporation of multiple types of terminal groups on dendrimer surfaces, several methodologies have been developed. These approaches allow for the introduction of various functional groups, enabling the synthesis of dendrimers with combined properties within a single molecule. One common method involves grafting two types of functions on the surface, either in a stochastic or precise manner, by sequentially reacting one functionality atop another in a stepwise synthesis [12]. However, the most intriguing approach involves the preparation of dendrimers with a multifunctional surface using dendrimeric structures composed of two dendrons (wedge-shaped dendrimeric units) through a central core coupling [13][14]. These structures are commonly referred to as Janus dendrimers [15], named after the Roman god of transitions and beginnings, depicted with two faces oriented in opposite directions. It is worth noting that Janus dendrimers have also been referred to by different names in the literature, such as surface block dendrimers [16] or di-block dendrimers [17].
Three main methodologies have been developed for the synthesis of Janus dendrimers, as depicted in Scheme 1. These methods involve either the reaction of two dendrons with complementary functions at the core level (method i); the sequential reaction of a first dendron with a well-defined core, followed by grafting a second dendron onto the remaining core functions (method ii); or the divergent growth of new branches from a focal point of a dendron (method iii).
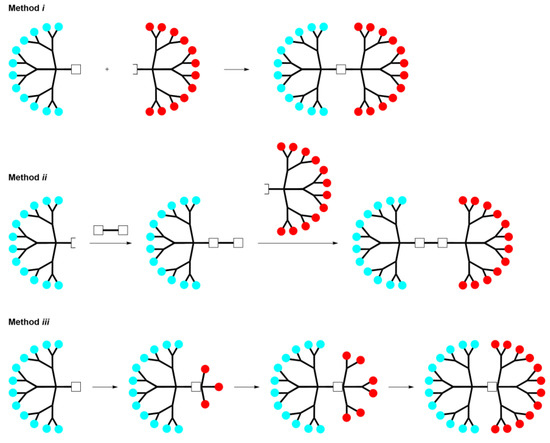
Scheme 1. Schematic representation of the main methods to synthesize Janus dendrimers with two functionalities at the surface.
Janus dendrimers have been the subject of interest since their first description by Fréchet et al. in 1991 [18]. In their pioneering work, a Janus dendrimer was synthesized through a convergent approach by coupling two poly(benzyl ether) (PBzE) dendrons. One dendron, owning a benzyl bromide group, was grafted onto a polyphenolic core through a monosubstitution reaction under basic conditions, resulting in an AB2-type core (one function different from the two others). The remaining unreacted phenol groups on the core were then reacted with other dendrons possessing different functionalities to complete the synthesis of the Janus dendrimer (Scheme 2).
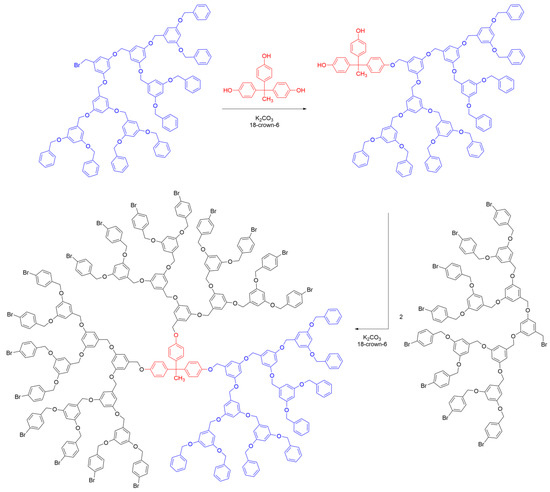
Scheme 2. Synthetic strategy for the preparation of a PBzE Janus dendrimer with a polyphenol core, based on method ii.
The synthesis of PBzE Janus dendrimers via substitution reactions involving phenols and benzylic halides has been widely employed in the literature. This approach has enabled the creation of Janus dendrimers with benzyl/carboxylate terminal groups or benzyl/nitrile functionalities [19]. These PBzE Janus dendrimers have found diverse applications, such as the formation of liquid membranes for organic/water interphase systems and the modification of polymer properties in terms of dipole moment [20] or glass transition temperatures [21], among others.
Interestingly, a polymerizable PBzE Janus dendrimer was also synthesized using a similar method, wherein a dendron terminated with oligoethylene (OEG) chains was reacted with a second dendron ended with benzyl groups and possessing a dibromobenzene unit at core (Figure 1). Later, this dibromobenzene group was reacted with diboronic acid in a polycondensation reaction to afford the desired polymeric structure [22].
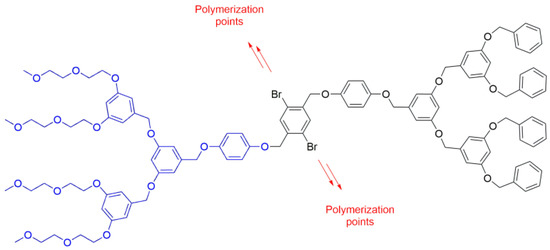
Figure 1. PBzE Janus dendrimer holding OEG chains on one side and bromine atoms at the core to be used as polymerization points, synthesized by method i.
In addition to PBzE dendrimers, classic poly(amidoamine) (PAMAM) structures have also been used for the synthesis of Janus dendrimers. For instance, Aoi et al. [23] demonstrated the synthesis of a Janus dendrimer by grafting a dendron bearing a primary amine core and terminated with sugar chains onto a second PAMAM dendron possessing phthaloyl groups on the surface through the formation of a urea linker by a reaction between a carbamoyl chloride and a primary amine (Scheme 3). They can be potentially used in the fields of supramolecular self-assembly or biology and biomedicine by means of binding surface sectors to proteins and DNA.
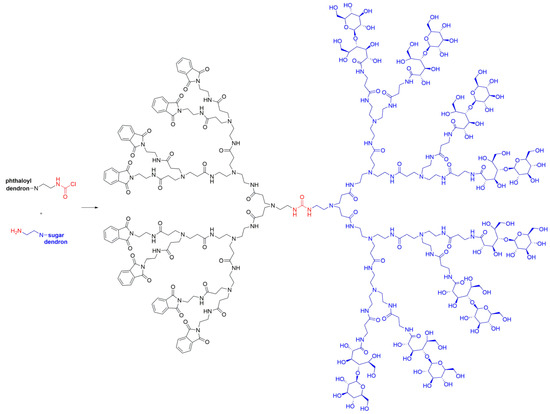
Scheme 3. PAMAM Janus dendrimer bearing phthaloyl groups on one side and sugars on the other, prepared by method i.
An intriguing Janus dendrimer was recently designed by Rosati et al. [24], employing a novel linker using “click” chemistry (Scheme 4). A dendrimer was obtained through a copper-catalyzed azide–alkylidene reaction where two dendrons — either with an azide or an acetylene group — were coupled through the formation of a triazole. The obtained Janus dendrimers displayed a tuning of their self-assembly properties by dispersion in aqueous solution and were targeted for applications such as the transport of gases or 19F NMR tracking in MRI (Magnetic Resonance Imaging).
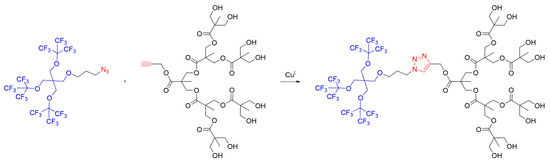
Scheme 4. Fluorinated Janus dendrimer synthesized by “click” reaction of two dendrons containing azide and acetylene moieties, based on method i.
The examples mentioned above provide only a glimpse into the extensive literature available on Janus dendrimers. These structures have been extensively explored, resulting in diverse compositions. For comprehensive reviews of the synthesis and properties of Janus dendrimers, refer to the work by Caminade et al. [14] and Căta et al. [25].
2. Phosphorus Dendrimers Built via Vinyl/Amine Coupling
The pioneering work of the group of Caminade and Majoral presented the first Janus dendrimer incorporating phosphorus atoms at each branching point. The dendrimer was synthesized by combining two dendrons, each possessing a complementary functionality at the core level. Initially, polyphosphorhydrazone (PPH) dendrons were synthesized via a divergent process, featuring a vinyl group at the core and various functional groups on the surface (Scheme 5a) [26]. The vinyl groups were subsequently converted to amine-terminated dendrons through a reaction with a diamine, allowing for subsequent coupling with another dendron. This synthetic approach helped the formation of Janus dendrimers by linking two dendrons together via vinyl/amine coupling, as illustrated in Scheme 6.
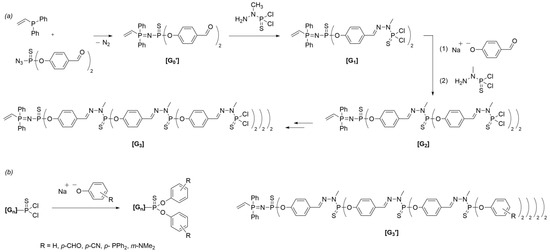
Scheme 5. (a) Synthesis of polyphosphorhydrazone dendrons bearing a vinyl group at the core up to the third generation. (b) Schematic representation of the surface functionalization of PPH dendrons, introducing aldehyde, nitrile, phosphine, or amino derivatives.
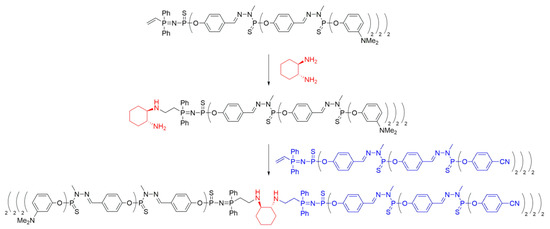
Scheme 6. Synthesis of polyphosphorhydrazone Janus dendrimers with a (1R,2R)-cyclohexane-1,2-diamine core, based on method ii.
In 2000, Maraval et al. [27] reported the synthesis of multidendritic systems based on the Michael addition coupling of amines to dendrons, featuring a vinyl core. The Janus dendrimers were created using an AB2-type monomer derived from thiophosphoryl chloride through a two-step process involving the introduction of two benzaldehyde moieties and an azide group. The reaction between the azide terminus and diphenylvinyldiphosphine yielded the desired vinyl core dendron with aldehyde functionalities on the surface. The growth of dendrons followed established methodologies for phosphorus dendrimer synthesis [28][29], involving a condensation reaction between the aldehyde groups and H2NN(Me)P(S)Cl2. Subsequent growth steps led to the dendrons’ formation, up to the third generation (Scheme 5a). The functionalization of the surface with various substituted phenolic salts resulted in fully characterized dendrons decorated with nitrile groups, phosphines, or amines (Scheme 5b). Dendrons were characterized at each synthetic step using 1H NMR, 31P NMR, 13C NMR, and IR techniques.
Further advancements in Janus dendrimer synthesis involved coupling two dendrons with vinyl groups using the aforementioned diamine method. The vinyl moiety was reacted with an excess of the corresponding diamine (ethylenediamine or (1R,2R)-cyclohexane-1,2-diamine) to obtain mono-addition products, which were subsequently reacted with a second dendron to yield the desired di-block dendrimers (Scheme 6). Evidence of successful core coupling was observed through an increased symmetry of the core, as confirmed by 13C NMR spectroscopy. This methodology allowed for the synthesis of various Janus dendrimers containing different functional groups (nitriles, phosphines, or amines) on their surfaces by combining two types of functional groups.
In 2006, Maraval et al. [30] employed the same vinyl/amine methodology to synthesize dendrimers with multi-charged surfaces. Specifically, they described the synthesis of polycationic, neutral, and polyanionic dendrons by incorporating ammonium tags, carboxylic acid groups, and carboxylates on the surface of the dendrons, respectively. The synthetic approach to obtain dendrons was previously described (refer to Scheme 5). The —P(S)Cl2 terminus of the vinyl dendrons was modified with the desired functionalities to generate polyionic systems, which could serve as promising candidates for biological applications [31][32] or layer-by-layer deposition (see later).
The synthesis of polycationic dendrons (Scheme 7) involved the reaction of the dendritic moieties with N,N-diethylethylenediamine through a substitution reaction to yield a tetraammonium-functionalized dendron. Hydrogen chloride generated during the process was quantitatively captured by the terminal amines. The progression of the reaction and the purity of the compounds were evaluated using 31P NMR, as the coupling with diethylethylenediamine resulted in a significant shift of the terminal phosphorus atom signal from δ = 63.4 ppm to δ = 73.4 ppm. It is important to emphasize the use of stoichiometric quantities of diethylethylenediamine to prevent Michael addition at the vinyl core. Subsequent treatment with NaOH produced the corresponding tetraamine-terminated dendrons. This strategy was employed for higher-generation dendrons, leading to ammonium- and amino-tagged dendrons up to the third generation.
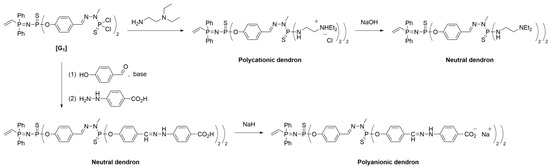
Scheme 7. Synthesis of polycationic, neutral, and polyanionic dendrons bearing a vinyl group at the core.
Polyanionic dendrons were synthesized through a three-step surface functionalization process (Scheme 7). The —P(S)Cl2 surface of the dendron was reacted with 4-hydroxybenzaldehyde under basic conditions, followed by a reaction with hydrazine benzoic acid to yield dendrons with carboxylic acid termini. Lastly, treatment with NaH resulted in the formation of carboxylate dendrons. The progression of the reactions was monitored using NMR techniques. The formation of the hydrazine derivative was confirmed by 1H NMR and IR, as indicated by the disappearance of aldehyde signals and the appearance of a new carboxylic acid peak. 31P NMR was used to confirm the integrity of the structure.
After synthesizing polyanionic, polycationic, and neutral dendrons, Janus dendrimers were prepared by coupling two dendritic moieties through their respective cores. The amine-terminated dendron was reacted with ethylenediamine, undergoing a Michael addition on the vinyl group at the dendron’s core to introduce the desired functionalized core. Subsequently, the coupling of two dendrons possessing vinyl and amino groups at the core was achieved in a biphasic THF/water system by simply combining the two dendrons at 70 °C (Scheme 8a). The progress of the reaction was monitored using 31P NMR, as the reaction of the vinyl group linked to the P=N—P=S moiety induced notable shifts in the NMR signals. In particular, the 31P NMR chemical shift for the P=N group moves from 14.7 ppm (d, 2Jpp = 28.0 Hz) in the dendron to 21.7 ppm (d, 2Jpp = 30.9 Hz) in the Janus dendrimer. This methodology enabled the synthesis of Janus dendrimers up to the third generation (Scheme 8b). In particular, Janus dendrimers of the hybrid generation were also obtained by combining dendrons of different generations [30].
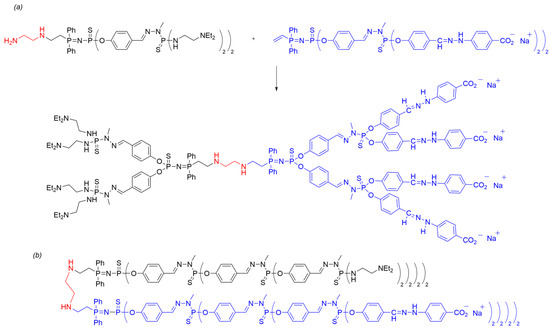
Scheme 8. (a) Synthesis of a polyanionic Janus dendrimer through amine/vinyl core–core coupling of two second-generation dendrons using method ii. (b) Linear representation of the third-generation Janus dendrimer with the same structural pattern.
Lastly, the authors demonstrated the applicability of the polycationic and polyanionic Janus dendrimers for layer-by-layer deposition. As previously shown by Kim et al. in 2005 [33], polyionic dendrimers can be employed to prepare nanotubes and microcapsules by alternately depositing layers of polyanionic and polycationic dendrimers. Preliminary results indicated the successful deposition of a polyanionic Janus dendrimer onto a silica surface pre-coated with a monolayer of 3-aminopropyl dimethylethoxy silane. Similar to the dendrimer depicted in Scheme 9, the deposition of anionic dendrimers onto a positively charged surface occurred through electrostatic interactions, resulting in a neutral surface. The amine terminus was subsequently quaternarized with methyl iodide as an alkylating agent to generate a positively charged surface, which was then covered with the polyanionic dendron part of the Janus dendrimer to assemble the second layer. By repeating this methodology, a thin film was constructed based on the quaternization and adsorption sequences, allowing for the deposition of up to four layers and the creation of nano-objects with potential applications in surface modification.
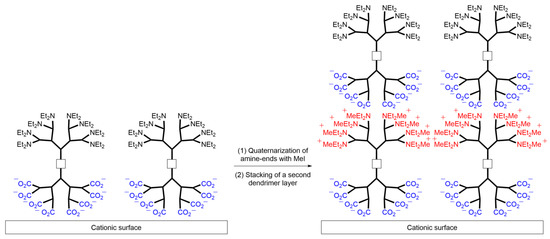
Scheme 9. Representation of the interactions between polyionic Janus dendrimers assembled in multilayer systems for applications in materials science.
The spectroscopic and microscopic characterization of these systems was conducted by Lee et al. [34]. UV–visible spectroscopy and atomic force microscopy (AFM) analyses confirmed the uniform formation of films over the entire substrate area, with a consistent increase in film thickness.
3. Synthesis of Dendrimers via Staudinger Reaction
The Staudinger reaction is exceptionally versatile in the realm of synthetic chemistry, primarily employed for the synthesis of iminophosphoranes through the reaction between an azide and a phosphine/phosphite [35]. Notably, these reactions typically occur under ambient conditions with simple reaction parameters, leading to high conversions and negligible by-products other than nitrogen gas. These unique characteristics have rendered the Staudinger reaction an outstanding methodology for the synthesis of intricate molecular structures [36][37][38].
In the context of dendrimer synthesis, the Staudinger reaction has found application as well. In 2001, Brauge et al. [39] reported the divergent synthesis of a phosphorus dendrimer, incorporating an iminophosphine in its branches. In this approach, an aldehyde-terminated monomer was reacted with a phosphorhydrazide dendron to yield a first-generation dendrimer through condensation reactions, resulting in a dendrimer possessing six terminal diphenylphosphine groups. Subsequently, phosphine groups were reacted with a functionalized azide using the Staudinger reaction, leading to the formation of the second-generation dendrimer (Scheme 10).
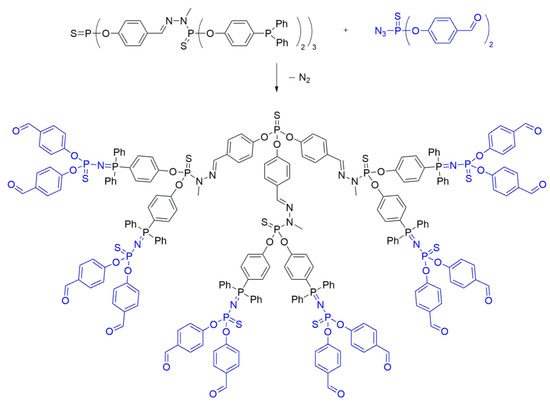
Scheme 10. Synthesis of a second-generation polyphosphorhydrazone (PPH) dendrimer by functionalization of the branches through Staudinger reaction.
Gottis et al. [40] employed a similar strategy for the coupling of dendrons to produce Janus dendrimers. They reported the synthesis of a hybrid carbosilane/PPH dendrimer through the formation of an iminophosphine bond. One dendron featured a thiophosphoryl azide group at the core, terminated with —P(S)Cl2 groups, while the other dendron possessed a phosphine moiety at the core and SiMe3 terminal groups. These two dendritic units were successfully coupled at room temperature, yielding the desired Janus dendrimer (Scheme 11). The reaction progress was monitored using 31P{1H} NMR, which revealed the formation of the iminophosphine bond and the generation of a P=N–P=S system through the appearance of distinct signals at δ = 51.9 ppm and δ = 19.8 ppm, corresponding to P=S and P=N, respectively. Janus dendrimers were synthesized up to the second generation using this method.
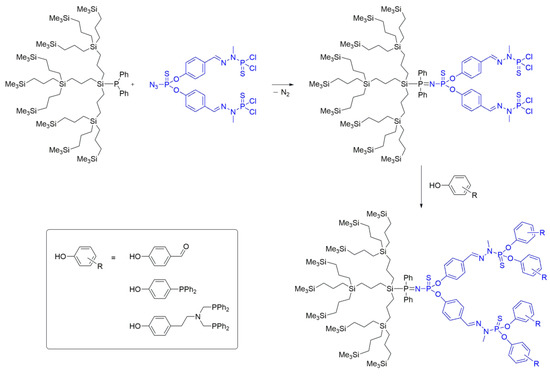
Scheme 11. Synthesis of a hybrid carbosilane/PPH Janus dendrimer (based on method i) via Staudinger reaction, and the functionality of its surface to accommodate aldehyde and phosphine groups.
The resulting Janus dendrimers were subsequently functionalized through the presence of —P(S)Cl2 groups on their surfaces. By subjecting the dendrimers to basic conditions, they were functionalized with 4-hydroxybenzaldehyde moieties, allowing for the reactivity of the system to be assessed. The substitution of the —P(S)Cl2 groups was monitored using NMR spectroscopy, which indicated a downfield shift from δ = 63.4 ppm (for unsubstituted —P(S)Cl2) to δ = 70.0 ppm upon the monosubstitution of the phosphorus atom with the phenol of interest to a —P(S)Cl(OR) system. This substitution pattern ultimately led to the disappearance of the signal at δ = 70.0 ppm, which was replaced by a singlet at δ = 60.7 ppm, corresponding to the fully substituted species. The same methodology was applied to incorporate other ligands, such as tyramine functionalized with two methylene bisdiphenylphosphines, demonstrating a consistent substitution pattern (Scheme 11). These novel phosphine-terminated carbosilane/PPH dendrimers were synthesized with the intention of their potential applications in catalysis in supercritical CO2, owing to the coordinating ability of the phosphine ligands present on the Janus dendrimer’s surface.
Interestingly, Maraval et al. [27] described the synthesis of a novel multiblock dendrimer by anchoring a dendron via Staudinger reaction to a previously obtained Janus dendrimer. It was synthesized using the methodology described in Scheme 6. Notably, a Janus dendrimer bearing terminal phosphine groups on one side was utilized for the synthesis of the mentioned multiblock system. As shown in Scheme 12, an azido dendrimer, which served as a building block for the preparation of the Janus dendrimers, was incorporated into the phosphine branches of the dendrimer, forming a P=N—P=S linkage. This demonstrated the versatility of the novel phosphine-ended Janus dendrimers in terms of their derivatization opportunities, yielding to the obtention of highly structural complex systems.
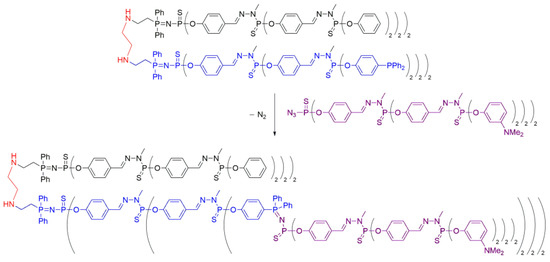
Scheme 12. Synthesis of a multiblock Janus dendrimer, with a dendron anchored at the surface of the Janus dendrimer via “click” Staudinger reaction, based on method iii.
In 2001, a novel methodology for the synthesis of core–core-built Janus dendrimers through Staudinger reaction was reported. The process involved alkylation and the subsequent desulfurization of a dendron P=N—P=S core, followed by a Staudinger reaction with a second desired dendron [41]. The initial dendron was constructed based on an AB2 core comprising two benzaldehyde groups and an azide [42]. These functional groups were later on modified through the Staudinger reaction with H2NN(Me)P(S)Cl2 and the desired phosphine, resulting in the formation of a first-generation dendritic moiety (Scheme 13).
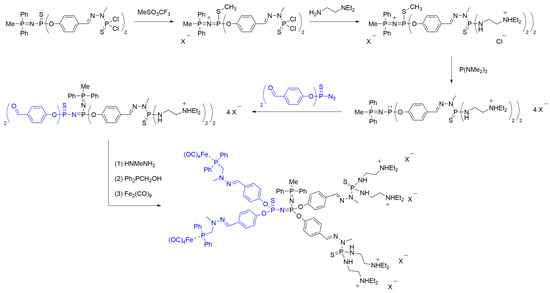
Scheme 13. Synthesis of a PPH Janus dendrimer via a novel desulfurization methodology and Staudinger reaction, following method iii.
The first-generation dendron was then employed in a novel approach to generate Janus dendrimers through a desulfurization reaction of the —P=S group at the core. As depicted in Scheme 13, the dendron was subjected to the alkylation of the sulfur atom at the core using methyl triflate. The success of this crucial step was confirmed by 31P NMR spectroscopy, which revealed the presence of two doublets at δ = 23.8 ppm and δ = 24.1 ppm (2JPP = 18.2 Hz), corresponding to the P=N—P—S—Me linkage, as well as a singlet at δ = 62.8 ppm for the terminal —P(S)Cl2 group. Subsequently, ionic exchange with NaBPh4 and a functionalization of the —P(S)Cl2 group with N,N-diethylethylenediamine were conducted to obtain a polycationic dendron, enabling solubility in a range of solvents, including water. The cationic dendron was further reacted with P(NMe2)3 to achieve desulfurization. This reaction was confirmed by 31P NMR, which displayed two doublets at δ = 144.2 ppm and δ = 10.3 ppm (2JPP = 31.6 Hz), corresponding to the core signals.
The activated desulfurized core served as the platform for coupling a second dendron through a Staudinger reaction with a monomer containing two benzaldehyde groups and a reactive azide. This coupling led to the formation of a novel P=N—P=N—P=S core linkage. The resulting Janus dendrimer was utilized for reaction with a di-iron nonacarbonyl compound. To achieve this, the aldehyde groups were reacted with excess methylhydrazine, followed by treatment with Ph2PCH2OH, yielding a dendrimer with terminal phosphine groups. Potentially, the prepared dendrimer containing phosphine groups at the surface could be used for the complexation of metals, such as palladium, for their potential applications in catalysis.
Finally, the desired Janus dendrimer with ammonium tags and incorporating iron complexes (Fe(CO)4) attached to a phosphine was obtained (Scheme 13). The formation of the Fe@dendrimer was confirmed by a significant deshielding of the phosphine signal, shifting from δ = -22.9 ppm to δ = 67.5 ppm [38].
4. Synthesis via Coupling of AB5 Cyclotriphosphazene Systems
AB5 compounds derived from the reactivity of hexachlorocyclotriphosphazene possess one function different from the five others. Such types of compounds have garnered significant attention in the scientific literature due to their facile syntheses [43]. The synthetic approach for generating these dendrons involves the asymmetric functionalization of the core [44], resulting in type compounds denoted as “A” and “B.” The “A” group serves as a linkage point for coupling with another dendron, while the “B” group represents the functional terminal groups. The synthetic methodologies employed to achieve the desired AB5 systems are diverse, and stem from two distinct approaches based on the substitution of the N3P3Cl6 core: either five P—Cl bonds are reacted with one reagent and then the last one with a second reagent, or vice versa, by first reacting one Cl with one reagent and the remaining five with a second reagent. The asymmetry of these compounds has led to the development of a great range of dendrons and dendritic structures, yielding to a plethora of compounds with different applications [45]. For a comprehensive review of the syntheses and applications of AB5 derivatives in the creation of phosphorus dendrons within the fields of biology, material science, catalysis, and sensors, refer to Zibarov et al. [45].
An intriguing example is the work described by Martinez-Ferrero et al. [46], wherein dendrimers were synthesized as hybrid optical sensors grafted onto mesoporous titania nanocomposites. An AB5 dendron was synthesized using N3P3Cl6 in a two-step process: first, the core was reacted with one equivalent of 4-hydroxybenzaldehyde to achieve the monosubstitution of one P—Cl bond, and second, the remaining five P—Cl bonds were reacted with maleimide-based phenolic chromophores. The successful substitution was confirmed through NMR and IR spectroscopy. The 1H and 13C NMR spectra exhibited multiple signals due to the asymmetric arrangement of the dendron branches around the core. The AB5 core was subsequently functionalized with H2NN(Me)P(S)Cl2 to enable the further modification of the dendritic structure. The terminal —P(S)Cl2 group was decorated with various phosphonate groups, known for their stability in forming complexes with metal oxides (Scheme 14).
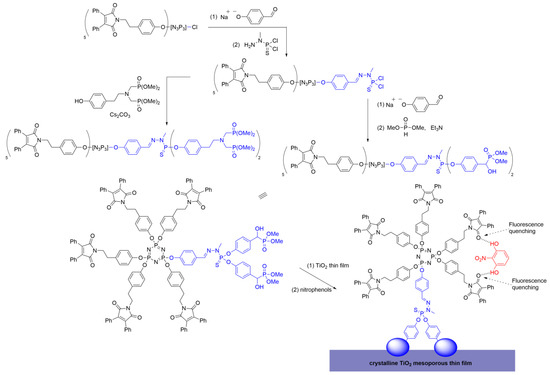
Scheme 14. Functionalization of a polyphosphorhydrazone Janus dendrimer with different phosphonates, and grafting to a TiO2 thin film, used as a fluorescent sensor for nitrophenols.
Considering the Janus dendrimers prepared, novel hybrid materials were created by combining the fluorescent properties of the maleimide derivative and anchoring the material onto a nanocrystalline mesoporous titania thin film. The primary attribute of these novel materials is their applicability as fluorescent probes. Simultaneously, titania films serve as excellent supports for grafting organic molecules due to their chemical composition, and their mesoporous cavities and optical properties do not interfere with those of the dendrimer. The designed systems exhibit a significant quenching efficiency toward phenolic OH groups, thus suggesting their potential application as detectors for hazardous phenolic compounds, as illustrated in Scheme 14.
Despite extensive research on the synthesis of dendrons using the AB5 derivative of cyclotriphosphazene, there are only two instances of employing these dendrons in the synthesis of Janus dendrimers, as described by Fuchs et al. in 2015 [47]. Utilizing the aforementioned methodology, two dendrons were synthesized, one containing five amino groups protected with tert-butyloxycarbonyl (Boc) groups and one carboxylic acid function, while the other contained five dansyl derivatives and one primary ammonium group (Scheme 15). Dansyl groups were chosen as the functional moiety for fluorescent labeling, as previously reported in the literature for fluorescent probes in dendrimers [48][49][50]. The synthesis of the Janus dendrimer proceeded through amide formation. Firstly, the carboxylic acid group of the first dendron was activated using TBTU (O-(benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate) in the presence of DIPEA (N,N’-diisopropylethylamine). Subsequently, the activated dendron was reacted with the ammonium salt of the second dendron under basic conditions to form an amide bond (Scheme 15a). Finally, the resulting Janus dendrimer was treated with an excess of trifluoroacetic acid in dichloromethane to remove the Boc protecting group, resulting in a dendrimer with dansyl groups on one side and ammonium tags on the other.
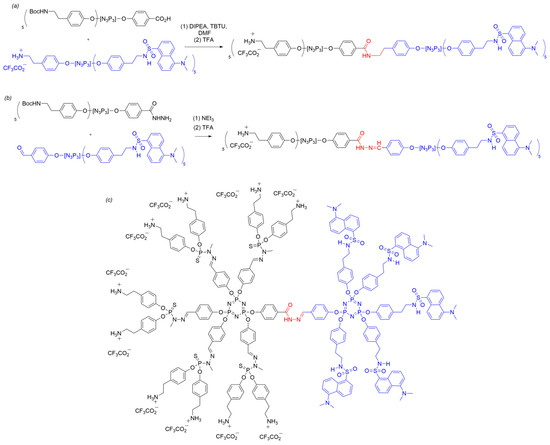
Scheme 15. Synthesis of Janus dendrimers (based on method i) bearing dansyl groups in one end and ammonium tags in the other through an (a) amide coupling; or (b) hydrazone linker; and (c) an expanded structure of a hybrid second-generation/first-generation Janus dendrimer with a hydrazone linker.
Another strategy to synthesize Janus dendrimers with dansyl and ammonium tags involved the use of a hydrazone bond (Scheme 15b). This approach was chosen because of the anticipated superior stability of hydrazones compared to that of amines in condensation reactions. For this purpose, two new dendrons were prepared: the first containing five Boc-protected primary amines and a hydrazide group, and the second containing five dansyl groups and one aldehyde group. The coupling of the two AB5 dendrons was achieved under basic conditions, resulting in the desired Janus dendrimer connected by a hydrazone linker. The obtained Janus dendrimer was again treated with trifluoroacetic acid to remove the Boc protecting group. The progress of the reaction was monitored by NMR and IR spectroscopy, observing the disappearance of the aldehyde signal and the formation of the hydrazone bond.
Both synthesized Janus dendrimers were designed for applications in the fields of material science [51] and biology [52][53][54], leveraging the presence of fluorescent dansyl groups and ammonium tags, which provide water solubility. The fluorescent properties of the dendrimers were evaluated in dioxane as the solvent, demonstrating similar absorption and emission maxima wavelengths with a substantial Stokes shift resulting from the presence of the dansyl groups. A minimal spectral overlap was observed between the absorption and emission spectra, a highly advantageous feature for future biological applications, as it enables an improved separation of the probe’s inherent light and the light scattered by the sample.
Of note, in 2011, Furer et al. conducted a density functional theory (DFT) study, as well as the IR and Raman spectra on the structure, of a dendron featuring five dansyl groups and a carbamate [55]. Computational studies involving structural optimization and normal mode analysis were performed, showing good agreement between the calculated geometrical parameters, harmonic vibrational frequencies, and experimental data. The calculations revealed that the dendron possesses a concave lens structure with a slightly non-planar cyclotriphosphazene core. Additionally, the intensity of the most prominent bands in the IR spectrum was reproduced by the calculations. Overall, the combined experimental and computational studies provided a detailed understanding of the dendron structure for the first time.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28145570
References
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2011.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic. Polym. J. 1985, 17, 117–132.
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005, 23, 1517–1526.
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and Hyperbranched Structures for Biomedical Applications. Eur. Polym. J. 2019, 119, 61–73.
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.P.; Caminade, A.-M.; Chaumonnot, A. Silica Functionalized by Bifunctional Dendrimers: Hybrid Nanomaterials for Trapping CO2. Eur. J. Inorg. Chem. 2016, 19, 3103–3110.
- Ali, B.M.; Kumar, K.A.; Sultan Nasar, A.S. Fifth Generation Polyurethane Dendrimers Decorated with Protected Amine, Free Amine and Blocked Isocyanate End Groups: Synthesis and Electrolytic Performance to Increase the Efficiency of Dye-Sensitized Solar Cell. ChemistrySelect 2019, 4, 12983–12991.
- Reek, J.N.H.; Arévalo, S.; van Heerbeek, R.; Kamer, P.C.J.; van Leeuwen, P.W.N.M. Dendrimers in Catalysis. Adv. Catal. 2006, 49, 71–151.
- Neumann, P.; Dib, H.; Caminade, A.-M.; Hey-Hawkins, E. Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. Int. Ed. 2015, 54, 311–314.
- Caminade, A.-M.; Laurent, R. Homogeneous Catalysis with Phosphorus Dendrimer Complexes. Coord. Chem. Rev. 2019, 389, 59–72.
- Thakare, S.; Shaikh, A.; Bodas, D.; Gajbhiye, V. Application of Dendrimer-Based Nanosensors in Immunodiagnosis. Colloids Surf. B 2022, 209, 112174.
- Satija, J.; Sai, V.V.R.; Mukherji, S. Dendrimers in Biosensors: Concept and Applications. J. Mater. Chem. 2011, 21, 14367–14386.
- Petriccone, M.; Laurent, R.; Turrin, C.-O.; Sebastián, R.M.; Caminade, A.-M. Specific Bifunctionalization on the Surface of Phosphorus Dendrimers Syntheses and Properties. Organics 2022, 3, 240–261.
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Janus-Type Dendrimers: Synthesis, Properties, and Applications. J. Mol. Liq. 2022, 347, 118396.
- Caminade, A.-M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” Dendrimers: Syntheses and Properties. New J. Chem. 2012, 36, 217–226.
- de Gennes, P.G. Nanoparticles and Dendrimers: Hopes and Illusions. Croat. Chem. Acta 1998, 71, 833–836. Available online: https://hrcak.srce.hr/132422 (accessed on 21 June 2023).
- Yanagimoto, Y.; Takaguchi, Y.; Tuboi, S. Novel Synthesis of Surface-Block Dendrimer Using Photocycloaddition of Anthryl Dendrons. Polym. J. 2006, 38, 1230–1236.
- Nierengarten, J.F.; Eckert, J.F.; Rio, Y.; Del Pilar Carrion, M.; Gallani, J.L.; Guillon, D. Amphiphilic Diblock Dendrimers: Synthesis and Incorporation in Langmuir and Langmuir-Blodgett Films. J. Am. Chem. Soc. 2001, 123, 9743–9748.
- Wooley, K.L.; Hawker, C.J.; Fréchet, J.M.J. Polymers with Controlled Molecular Architecture: Control of Surface Functionality in the Synthesis of Dendritic Hyperbranched Macromolecules Using the Convergent Approach. J. Chem. Soc. Perkin Trans. 1 1991, 5, 1059–1076.
- Hawker, C.J.; Wooley, K.L.; Fréchet, J.M.J. Unimolecular Micelles and Globular Amphiphiles: Dendritic Macromolecules as Novel Recyclable Solubilization Agents. J. Chem. Soc. Perkin Trans. 1 1993, 12, 1287–1297.
- Wooley, K.L.; Hawker, C.J.; Fréchet, J.M.J. Unsymmetrical Three-Dimensional Macromolecules: Preparation and Characterization of Strongly Dipolar Dendritic Macromolecules. J. Am. Chem. Soc. 1993, 115, 11496–11505.
- Wooley, K.L.; Hawker, C.J.; Pochan, J.M.; Fréchet, J.M.J. Physical Properties of Dendritic Macromolecules: A Study of Glass Transition Temperature. Macromolecules 1993, 26, 1514–1519.
- Bo, Z.; Rabe, J.P.; Schlüter, A.D. A Poly(para-phenylene) with Hydrophobic and Hydrophilic Dendrons: Prototype of an Amphiphilic Cylinder with the Potential to Segregate Lengthwise. Angew. Chem. Int. Ed. 1999, 38, 2370–2372.
- Aoi, K.; Itoh, K.; Okada, M. Divergent/Convergent Joint Approach with a Half-Protected Initiator Core To Synthesize Surface-Block Dendrimers. Macromolecules 1997, 30, 8072–8074.
- Rosati, M.; Acocella, A.; Pizzi, A.; Turtù, G.; Neri, G.; Demitri, N.; Raffaini, G.; Donnio, B.; Zerbetto, F.; Baldelli Bombelli, F.; et al. Janus-Type Dendrimers Based on Highly Branched Fluorinated Chains with Tunable Self-Assembly and 19F Nuclear Magnetic Resonance Properties. Macromolecules 2022, 55, 2486–2496.
- Căta, A.; Ienașcu, I.M.C.; Ştefănuț, M.N.; Roșu, D.; Pop, O.R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics 2023, 15, 589.
- Maraval, V.; Laurent, R.; Donnadieu, B.; Mauzac, M.; Caminade, A.-M.; Majoral, J.P. Rapid Synthesis of Phosphorus-Containing Dendrimers with Controlled Molecular Architectures: First Example of Surface-Block, Layer-Block, and Segment-Block Dendrimers Issued from the Same Dendron. J. Am. Chem. Soc. 2000, 122, 2499–2511.
- Maraval, V.; Laurent, R.; Merino, S.; Caminade, A.-M.; Majoral, J.P. Michael-Type Addition of Amines to the Vinyl Core of Dendrons—Application to the Synthesis of Multidendritic Systems. Eur. J. Org. Chem. 2000, 21, 3555–3568.
- Launay, N.; Caminade, A.-M.; Majoral, J.P. Synthesis and Reactivity of Unusual Phosphorus Dendrimers. A Useful Divergent Growth Approach Up to the Seventh Generation. J. Am. Chem. Soc. 1995, 117, 3282–3283.
- Galliot, C.; Prévoté, D.; Caminade, A.-M.; Majoral, J.P. Polyaminophosphines Containing Dendrimers. Syntheses and Characterizations. J. Am. Chem. Soc. 1995, 117, 5470–5476.
- Maraval, V.; Maraval, A.; Spataro, G.; Caminade, A.-M.; Majoral, J.P.; Kim, D.H.; Knoll, W. Design of Tailored Multi-Charged Phosphorus Surface-Block Dendrimers. New J. Chem. 2006, 30, 1731–1736.
- Blanzat, M.; Turrin, C.O.; Aubertin, A.M.; Couturier-Vidal, C.; Caminade, A.-M.; Majoral, J.P.; Rico-Lattes, I.; Lattes, A. Dendritic Catanionic Assemblies: In Vitro Anti-HIV Activity of Phosphorus-Containing Dendrimers Bearing Galβ1cer Analogues. ChemBioChem 2005, 6, 2207–2213.
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Béranger, F.; Caminade, A.-M.; Meunier, B.; Dormont, D.; Majoral, J.P.; Lehmann, S. Cationic Phosphorus-Containing Dendrimers Reduce Prion Replication Both in Cell Culture and in Mice Infected with Scrapie. J. Gen. Virol. 2004, 85, 1791–1799.
- Kim, B.S.; Lebedeva, O.V.; Kim, D.H.; Caminade, A.-M.; Majoral, J.P.; Knoll, W.; Vinogradova, O.I. Assembly and Mechanical Properties of Phosphorus Dendrimer/Polyelectrolyte Multilayer Microcapsules. Langmuir 2005, 21, 7200–7206.
- Lee, O.J.; Maraval, V.; Caminade, A.-M.; Chung, K.; Lau, K.H.A.; Shin, K.; Majoral, J.P.; Knoll, W.; Kim, D.H. Layer-by-Layer Self-Assembly of Bisdendrons: An Unprecedented Route to Multilayer Thin Films. Macromol. Res. 2016, 24, 851–855.
- Staudinger, H.; Hauser, E. Über Neue Organische Phosphorverbindungen IV Phosphinimine. Helv. Chim. Acta 1921, 2, 861–887.
- Gololobov, Y.G.; Zhmurova, I.N.; Kasukhin, L.F. Sixty Years of Staudinger. Tetrahedron 1981, 37, 437–472.
- Gololobov, Y.G. Recent Advances in the Staudinger Reaction. Tetrahedron 1992, 48, 1353–1406.
- Fresneda, P.M.; Molina, P. Application of Iminophosphorane-Based Methodologies for the Synthesis Of Natural Products. Synlett 2004, 1, 1–17.
- Brauge, L.; Magro, G.; Caminade, A.-M.; Majoral, J.P. First Divergent Strategy Using Two AB2 Unprotected Monomers for the Rapid Synthesis of Dendrimers. J. Am. Chem. Soc. 2001, 123, 6698–6699.
- Gottis, S.; Rodriguez, L.I.; Laurent, R.; Angurell, I.; Seco, M.; Rossell, O.; Majoral, J.P.; Caminade, A.-M. Janus Carbosilane/Phosphorhydrazone Dendrimers Synthesized by the ‘Click’ Staudinger Reaction. Tetrahedron Lett. 2013, 54, 6864–6867.
- Maraval, V.; Sebastián, R.M.; Ben, F.; Laurent, R.; Caminade, A.-M.; Majoral, J.P. Varying Topology of Dendrimers—A New Approach toward the Synthesis of Di-Block Dendrimers. Eur. J. Inorg. Chem. 2001, 7, 1681–1691.
- Mitjaville, J.; Caminade, A.-M.; Mathieu, R.; Majoral, J. New Synthetic Strategies for Phosphorus-Containing Cryptands and the First Phosphorus Spherand Type Compound. J. Am. Chem Soc. 1994, 116, 5007–5008.
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822.
- Maraval, V.; Caminade, A.-M.; Majoral, J.P.; Blais, J.C. Dendrimer Design: How to Circumvent the Dilemma of a Reduction of Steps or an Increase of Function Multiplicity? Angew. Chem. Int. Ed. 2003, 42, 1822–1826.
- Zibarov, A.; Oukhrib, A.; Catot, J.A.; Turrin, C.O.; Caminade, A.-M. AB5 Derivatives of Cyclotriphosphazene for the Synthesis of Dendrons and Their Applications. Molecules 2021, 26, 4017.
- Martínez-Ferrero, E.; Franc, G.; Mazères, S.; Turrin, C.O.; Boissière, C.; Caminade, A.-M.; Majoral, J.P.; Sanchez, C. Optical Properties of Hybrid Dendritic-Mesoporous Titania Nanocomposite Films. Chem. Eur. J. 2008, 14, 7658–7669.
- Fuchs, S.; Pla-Quintana, A.; Mazères, S.; Caminade, A.-M.; Majoral, J.P. Cationic and Fluorescent “Janus” Dendrimers. Org. Lett. 2008, 10, 4751–4754.
- Branchi, B.; Ceroni, P.; Bergamini, G.; Balzani, V.; Maestri, M.; Van Heyst, J.; Lee, S.K.; Luppertz, F.; Vögtle, F. A Cyclam Core Dendrimer Containing Dansyl and Oligoethylene Glycol Chains in the Branches: Protonation and Metal Coordination. Chem. Eur. J. 2006, 12, 8926–8934.
- Fuchs, S.; Otto, H.; Jehle, S.; Henklein, P.; Schlüter, A.D. Fluorescent Dendrimers with a Peptide Cathepsin B Cleavage Site for Drug Delivery Applications. Chem. Commun. 2005, 1, 1830–1832.
- Fuchs, S.; Kapp, T.; Otto, H.; Schöneberg, T.; Franke, P.; Gust, R.; Schüter, A.D. A Surface-Modified Dendrimer Set for Potential Application as Drug Delivery Vehicles: Synthesis, In Vitro Toxicity, and Intracellular Localization. Chem. Eur. J. 2004, 10, 1167–1192.
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.-M.; Majoral, J.P.; Knoll, W. Functional Quantum-Dot/Dendrimer Nanotubes for Sensitive Detection of DNA Hybridization. Small 2008, 4, 566–571.
- Mongin, O.; Krishna, T.R.; Werts, M.H.V.; Caminade, A.-M.; Majoral, J.P.; Blanchard-Desce, M. A Modular Approach to Two-Photon Absorbing Organic Nanodots: Brilliant Dendrimers as an Alternative to Semiconductor Quantum Dots? Chem. Commun. 2006, 8, 915–917.
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournié, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.-M.; et al. Tailored Control and Optimisation of the Number of Phosphonic Acid Termini on Phosphorus-Containing Dendrimers for the Ex-Vivo Activation of Human Monocytes. Chem. Eur. J. 2008, 14, 4836–4850.
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Métivier, P.; Bacquet, G.; Fournié, J.J.; Caminade, A.-M.; et al. Multiplication of Human Natural Killer Cells by Nanosized Phosphonate-Capped Dendrimers. Angew. Chem. Int. Ed. 2007, 46, 2523–2526.
- Furer, V.L.; Vandyukova, I.I.; Vandyukov, A.E.; Fuchs, S.; Majoral, J.P.; Caminade, A.-M.; Kovalenko, V.I. DFT Study of Structure, IR and Raman Spectra of the Fluorescent “Janus” Dendron Built from Cyclotriphosphazene Core. J. Mol. Struct. 2011, 1005, 25–30.
This entry is offline, you can click here to edit this entry!
