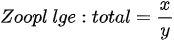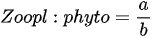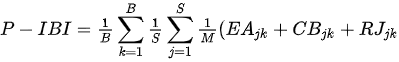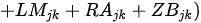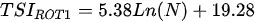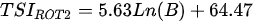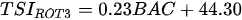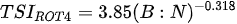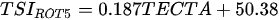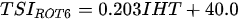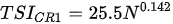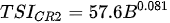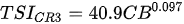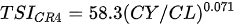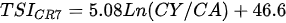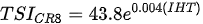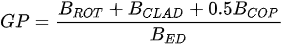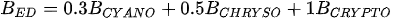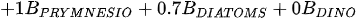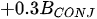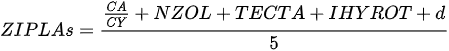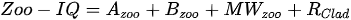Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
For the sustainable use of lake ecosystem services—water resources, aquatic habitats for biodiversity conservation, and aesthetic values as waterfront space—ecosystem health assessments using biota are implemented as important national environmental monitoring projects. Zooplankton play a key role as an important linkage in the material circulation as secondary producers in lake ecosystems. At the same time, they influence the composition and biomass of other communities through biological interactions.
- zooplankton
- multi-metric index
- biotic indices
- biomass
1. The Role of Zooplankton in Lake Ecosystems
Lake ecosystems are enclosed areas with a long residence time and endogenous organic matter produced by macrophytes, sessile algae, and phytoplankton [1]. Externally introduced organic matter and pollutants are circulated within the lake through biological food webs [2]. Therefore, information related to energy and material flow, acquired by identifying changes in biological interactions among lake organisms within the food web, can be effectively used to represent the health status of the overall lake ecosystem [3].
Within lake food webs, zooplankton contains key species, which have a major influence on ecosystem function and stability. Zooplankton are a food source not only for planktivorous fish species, but also for juveniles of most fish species, including piscivorous fish, which are top predators, as well as grazers that control phytoplankton and bacterial populations [4][5][6]. Therefore, identifying changes in zooplankton communities (individual size, biomass, population, community structure, etc.) within lake ecosystems is an important task for effective lake management [7][8].
In a broad sense, zooplankton may include various Protozoa, such as Ciliophora and Amoebozoa, but zooplankton that can be easily quantified using a 60 μm zooplankton net—a common collecting tool—includes Rotifera, Cladocera, and Copepoda [9]. Rotifera are the most common taxa, appearing in almost all lake and river ecosystems. They have a wide variety of forms, including thick-shelled, non-shelled, and soft-shelled species [10]. Rotifera have potential as biological indicators of a lake’s trophic state, as they have a short life cycle and respond rapidly to environmental changes, making them well-adapted to dynamic environments [11][12]. Cladocera are Crustacea that swim using long tentacles and branched antennae that extend like arms [13]. They consume phytoplankton through filter-feeding; at the same time, they are a preferred food source for planktivorous fish. Through these biological interactions, Cladocera occupies an intermediate trophic level in the lake food web and plays an important role in material and energy circulation and water quality in lake ecosystems [14][15][16][17][18]. There are approximately 2800 freshwater Copepoda species, over a total of 13,000 described species [19]. Copepoda exhibit a very wide range of lifestyles, from predation to parasitism and, like Cladocera, serve as a food source for planktivorous fish. As such, these species also play an important role in material and energy circulation in aquatic ecosystems [20]. As mentioned above, zooplankton vary in size and swimming ability, are found at various trophic levels, and include species with different feeding characteristics and food selectivity. The distribution of zooplankton is influenced by abiotic limits, methods and means of distribution, and biological interactions [21][22][23][24]. In the event of an ecosystem disturbance, the structure of the community changes rapidly, leading to a simplification of the community and the disappearance of species. However, the resting ability of all zooplankton allows them to remain inactive in each lake and reestablish themselves in the water column when favorable conditions return. Using these changes in community composition, it becomes possible to effectively evaluate the health of the lake ecosystem [12][25][26][27].
Owing to their sensitivity to environmental changes and their important role in the freshwater food web, zooplankton species have been used effectively to assess the trophic state of water bodies [12][16][23][28]. In aquatic ecosystem monitoring, zooplankton indices can be used to indicate the overall health of the system. Information about the lake environment related to energy flow and material flow through biological interactions within the food web can be identified through zooplankton, (1) the biomass of important secondary producers; (2) changes in the microbial food web and grazing food web as the zooplankton community changes; (3) the quality improvement of total organic C circulating within the aquatic ecosystem food web; and (4) ecological shifts as a result of climate change or toxic substances [29][30][31][32][33][34]. In addition, for biomanipulated lakes where planktivorous were removed to maximize the zooplankton grazing effect for water quality maintenance, indices regarding zooplankton composition (indices representing the recovery of Cladocera, particularly genus Daphnia) can be used as evidence to confirm the success of biomanipulation [35][36].
2. Zooplankton Communities’ Response Patterns to Environmental Change
Studies on zooplankton community-based indices to diagnose ecological and environmental status of lakes have long been conducted [4][16]. Broadly, zooplankton species are used as indicators of (i) eutrophication in a comprehensive sense, including water quality; (ii) watershed development and land use; and (iii) biological interactions (bottom-up and top-down pressures). Changes in zooplankton communities are measured in terms of the abundance, richness, and biomass of specific taxa. In recent studies on zooplankton indicators, the use of biomass has become more common [12][26][27][28].
The responses of zooplankton communities to lake eutrophication can be classified in a taxon-specific manner. Zooplankton community characteristics suggested to be inversely proportional to the progress of eutrophication include species richness, average size, and the Calanoida/(Cyclopoida + Cladocera) abundance ratio [21]. Potential indicator species that appear in eutrophic lakes and increase in abundance with eutrophication include Keratella cochlearis f. tecta, K. tropica, Brachionus budapestinensis, and B. calyciflorus. In contrast, the abundances of Conochilus unicornis, C. dossuarius, C. coenobasis, and Ascomorpha ovalis increase as the trophic state is lowered, and these species have been suggested as oligotrophic indicators [11][21][37].
Zooplankton community indicators that are reportedly inversely proportional to the total P concentration in a water body include species richness, body weight of planktonic Crustacea, the biomass ratio of Daphnia spp. among Crustacea, and the biomass and abundance of Calanoida among Copepoda [38]. Factors suggested to be proportional to the total P concentration include the proportions of biomass and the abundance of Cyclopoida among Copepoda. In an analysis of zooplankton samples from 146 lakes in the northeastern United States, the Ca concentration (hardness) of the water appeared to be proportional to the abundance of large Cladocera (e.g., Daphnia pulex, D. pulicuria, D. schodleri, and D. galeata mendotae), and inversely proportional to that of small Rotifera (<0.2 mm) [39].
It has also been suggested that increases in the total P concentration, Chlorophyll a concentration, and anthropogenic land use in a watershed can lead to a decrease in the species richness of Calanoida and large Cladocera, and the abundance of Daphnia pulicaria, a large crustacean. Increases in the abundance and richness of small Cladocera (e.g., Bosminidae, Chidoridae), Rotifera, Cyclopoida, Nauplius and Skistodiaptomus pallidus have also been reported [24][40].
In the western United States, the biomass of large Cladocera (D. pulex complex) and Cyclopoida (Diacyclops thomasi) reportedly increases as lakes become deeper and cooler, and with an increase in unproductive land use and forested catchment in the watershed [41]. The biomass of small Cladocera (Daphnia retrocurva, Diaphanosoma spp., Cydorus sphaericus) and Cyclopoida (Tropocyclops prasinus) increases as watershed land becomes more productive and is affected by agriculture [41]. It has also been suggested that the body length of Cladocera and the biomass of Daphnidae decrease as water temperature increases and latitude decreases [41].
In terms of biological interactions, the specialist Cladocera and Calanoida species, commonly found in eutrophic lakes, were identified [24]. The biomass of Rotifera and Cyclopoida reportedly increases as bottom-up pressures increase, including both direct and indirect pressures caused by nutrient salts (total P) and Chlorophyll a, while the biomass of Calanoida and Cladocera decreases [42].
The average body weight of Cladocera and the biomass ratio of zooplankton/phytoplankton reportedly decrease as the biomass of planktivorous fish increases [38]. At low latitudes, the predation pressure on zooplankton by planktivorous fish, such as salmon (Oncorhynchus spp.), lake trout (Salvelinus spp.), and gizzard shad (Dorosoma cepedianum), is high, affecting the size and biomass of Cladocera and Copepoda [41] .
In previous studies related to zooplankton indicators, regardless of region or disturbance factors, the abundance or biomass of large Cladocera, Calanoida Copepoda, decreased as disturbances increased (total P concentration, predation pressure of fish, commercial land use in watersheds, etc.). In contrast, the abundance or biomass of small Cladocera, Rotifera, and Cyclopoida Copepoda showed an increasing trend.
Although there is a common zooplankton community that responds to disturbance, zooplankton species may respond differently, depending on the area of occurrence and the disturbance. When developing a lake health evaluation index using the results from previous studies, an understanding of the zooplankton species inhabiting the target area is needed before a “good” index can be selected [43].
Alongside studies of changes in zooplankton communities in response to environmental change, research on group-specific separations of response factors and the formation of formulae have also been conducted. In a previous study of the degree of eutrophication of a lake ecosystem, the number of species of specific zooplankton taxa (Rotifera, Copepoda, and Cladocera) and a eutrophication index were used together. Index values < 0.2 indicated oligotrophic lakes, greater than 0.2 and less than 1 were mesotrophic, greater than 1 and less than 4 were eutrophic, and greater than 4 indicated hypertrophic lakes [44].
In summary, assessments of zooplankton community changes to evaluate the health of lake ecosystems have been conducted, but with variations in the zooplankton indicators, type of environmental disturbance, and zooplankton characteristics used. Inconsistencies in evaluation results using specific taxa have also been reported, with conflicting trends in the abundance or species composition [10]. Recently, a more comprehensive metric to evaluate a specific lake has been developed.
3. Zooplankton Indices for Freshwater Ecosystem Health Assessment
Recently, many multi-metric indices (MMI) for freshwater (especially lake) ecosystem health assessments have been developed and used. The multi-metric method using an integrated index responding to multiple co-varying stressors provides a more comprehensive assessment, and increases confidence in assessment [45][46]. This is obtained by eliminating the possible dilemmas arising when only a single index or assemblage attributed to the different types of indices responds differently to diverse stressor types [47].
The European ECOFRAME project (for the ecological quality and functioning of shallow lake ecosystems with respect to the needs of the European Water Framework Directive 2003), through the development of a zooplankton index for assessing shallow lakes, has shown that the ratio of specific taxonomic groups in zooplankton communities is more effective than using absolute values (e.g., number of species and abundance) for ecosystem assessment [48]. In this index, the zooplankton species were categorized according to their body size, and simple coefficients calculated based on the proportion of large-bodied zooplankton biomass were used. Zooplankton body size is an important factor in determining their susceptibility to fish predation and a prey selection spectrum for phytoplankton assemblages. In ecologically healthy lakes, riparian aquatic plant communities function as effective shelters for large zooplankton, which can shed predatory pressure of fish [49]. Thus, the proportion of large Cladocera is higher in ecologically healthy lakes than in unhealthy lakes. species between 0.2 and 5 mm (Diaphanosoma, Moina, Leydigia leydigii, Holopedium gibberum, and Simocephalus vetulus) were classified as large Cladocera. The large bodied-zooplankton category generally includes Cladocera and Copepoda larger than 0.48 mm in size; specifically, among Cladocera species, genera Ceriodaphnia, Moina, Diaphanosoma, and Daphnia are included, while Bosmina, Alona, and Chydorus are excluded [50]. As zooplankton are grazers of phytoplankton, the “zooplankton biomass/Chlorophyll a concentration” equation was created to calculate the predation pressure of zooplankton, and the phytoplankton biomass was replaced by the Chlorophyll a concentration. This index calculates the impact of zooplankton on phytoplankton as the ratio of producers to primary consumers [48].
Kane et al. [25] presented the Planktonic Index of Biotic Integrity(P-IBI) that can assess the health of Lake Erie at the catchment scale using appropriate metrics selected through factor analysis among candidate metrics suggested in the available literature. The study utilized seasonally varying scores to represent different environmental conditions throughout the year. The mean site score for each year was determined by summing the individual planktonic metric scores for June, July, and August and dividing by the number of metrics. Furthermore, the basin mean score was calculated by summing the average site scores and dividing by the number of sites. Subsequently, the lake-wide plankton IBI scores were obtained by summing the basin average scores and dividing by the number of basins. This index demonstrates a strong correspondence with traditional measures of lake nutrient status, reflecting the degree of eutrophication. The ratio of the total June phytoplankton biomass index scores (CBjk) directly uses the percentage values of Cyanobacteria abundance, Anabaena, Aphanizomonon, and Microcystis, which reduce water quality as undesirable algae, and the usefulness of the phytoplankton community as a food source. The Limnocalanus macrurus abundance metric score (LMjk) in July and the (Calanoida/[Cladocera + Cyclopoida]) ratio (RJjk) in June decline together, indicating the food environment for the zooplankton community had deteriorated. Individual metrics using different plankton groups can provide information on benefit use impairment (BUI), which indicates the degree of eutrophication at different trophic levels. BUI can explain the damage to water resources by humans and assess the degree of impact on human health (e.g., water restriction), ecosystem function, or both [25].
Ejsmont-Karabin [12][28] developed an index to calculate TSIROT and TSICR (Indices of the trophic state of lakes), which are health status values of lakes using Rotifera and Crustacea. Through statistical analysis, the relationship between TSI(SD+CHL)—which is the average of TSISD (trophic state index calculated by transparency) and TSICHL (trophic state index calculated by the concentration of Chlorophyll a)—and zooplankton indicators that can indicate the trophic state of the lake (e.g., Rotifera abundance, Crustacea abundance, and Cyclopoida biomass) was identified, and a relational equation was presented. This equation indicates the trophic state of the lake using zooplankton. Lakes with TSIROT and TSICR values <45 were presented as oligotrophic, 45–55 as mesotrophic, 55–65 as eutrophic, and >65 as hypertrophic. Keratella cochlearis, which is the most common rotifer that appears in most lakes, shows a tendency to increase the abundance of Keratella cochlearis f. tecta type, in which the posterior spine disappears when the lake is eutrophic [51][52]. The following data are presented as equations that can explain the trophic state of lakes: percentage of form tecta in the population of Keratella cochlearis, Rotifera numbers, Rotifera biomass, percentage of bacterivores in the total number of Rotifera, ratio of biomass to number, percentage of species indicative of high trophy in the indicative Rotifera group’s number, number of Crustacea, biomass of Cyclopoida, percentage of Cyclopoida biomass in the total biomass of Crustacea, ratio of the Cyclopoida biomass to the biomass of Cladocera, ratio of Cyclopoida to Calanoida numbers, and percentage of species indicative of high trophy in the indicative number of Crustacea [12][28].
A more specific index focusing on the biological interaction between zooplankton and phytoplankton, the grazing potential (GP) index, combining the zooplankton and phytoplankton indices, has been proposed [26]. The GP value, formed using the sum of specific species, provides important information on food web function in an easy and cost-effective manner by combining zooplankton and phytoplankton metrics. Lakes with high GP values show that large Cladocera and Copepoda occupy a large proportion of the total zooplankton biomass. However, lakes with low GP values show a trend toward increased phytoplankton biomass, or increased population density of small zooplankton. The variables used in GP are identified at the genus level rather than at the species level, making it easier to identify and calculate than an index that uses species-level information. In the BED value calculation, a total phytoplankton index, a multiple (very good [1]; very bad [0]) was set in front of the variable to account for the relative edibility of phytoplankton [26].
On the other hand, index based on local biological assemblages and their interactions with environmental variables also has been developed. Ochocka [43] presented the Zooplankton Index of Polish Lakes’ Assessment (ZIPLAs), which showed the strongest correlation with transparency. Of the 31 candidate metrics, five were selected for use in the index, showing the strongest correlations with TP, TN, transparency, and PCATOT (a cumulative nutrient load index calculated based on principal component analysis). The ecologically healthiest reference lakes were those that had no sources of pollution entering the water body, had the highest water quality status according to existing data, and had at least 80% natural land use within the watershed. Additionally, the dividing values of high (H), good (G), normal (M), poor (P), and very poor (B) indices were 75%, 50%, and 25% of the reference lake. CA/CY is the second most strongly correlated metric with TP for the ratio of Calnoida to Cyclopoida individual numbers, and the value of this index decreases with increasing eutrophication. Based on these results, it is clear that Calanoida prefer oligotrophic lakes. Zooplankton abundance (NZOL) is a commonly used index for assessing the trophic state of lakes [53][54][55]. This index is easy to calculate, decreases as TP concentrations increase, and is highly correlated with the nutrient status of the lake. The percentage of tecta in the population of Keratella cochlearis (TECTA) is an available indicator of the ecological status of lakes, particularly for lakes where Rotifera dominate and Cladocera and Copepoda are rare. Keratella cochlearis f. tecta, K. quadrata, Pompholyx sulcata, Filinia longicata, Anualeopsis fissa, Trichocerca pulchella, Brachionus undularis, and Brachionus dirsicornis were used as indicative species of high trophic levels. Rotifera species indicative of high trophic levels in the group that frequently occur in high-nutrient lakes can differ from country to country. Therefore, a list of national and regional indicator species should be used when assessing lakes using this index. The two diversity indices were the Shannon–Weaver index and the Margalef index; however, of the two indices, the Margalef (d) index, which represents the number of species relative to the total number of individuals, in contrast to the Shannon–Weaver index, which has a statistically highly significant correlation with environmental variables. Consequently, ZIPLAs decreased as the pollution level of the lakes increased [43].
Stamou [27] presented the Zoo-IQ index, which includes zooplankton biomass and body size information. The metrics used in this index are the total abundance of zooplankton (Azoo) and total dry biomass of zooplankton (Bzoo), which are indices of the response of zooplankton to phytoplankton. Under eutrophic conditions characterized by increased nutrient content and bottom-up pressure, Azoo and Bzoo exhibit a tendency to rise, potentially impacting zooplankton population growth. The morphometric mean size of zooplankton (MWzoo) provides insights into the functioning of the pelagic food web in lakes, where the presence of large Cladocera (particularly Daphnia) and Calanoida can effectively control the entire size range of phytoplankton and even induce clear water phases. The mean size was not measured directly, but was calculated as the ratio of dry biomass to abundance. The ratio of large Cladocera to total Cladocera abundance (Rclad) serves as an estimate of the changes in dominance among different functional groups, determined by Cladocera feeding mode. However, Rclad and MWzoo decrease as eutrophication processes, reflecting changes in zooplankton groups’ domination patterns, with small-bodied species dominating in eutrophic lakes with Cyanobacteria blooms, but also the predation pressure by fish on large- bodied species. The metric value of the healthiest lake in the dataset was set as the reference value; the metric value was rated as good (5) if it was similar to the reference value; normal (3) if different from the reference value; and poor (1) if substantially different from the reference value. The sum of all metric scores was set as the Zoo-IQ value. This index is the result of synthesizing various stress factors, and can be used as an index to evaluate the decline in the general lake status [27].(Table 1)
Table 1. Zooplankton metrics developed for lake health assessment.
|
Zooplankton Metrics |
Description |
Parameter |
Ref. |
|
|
Ratio of large Cladocera frequently appearing in healthy lake |
x = Large Cladocera (>0.5 mm) individual number
|
[48] |
|
|
Effects of zooplankton predation on phytoplankton |
a = Cladocera and copepod biomassb = Chlorophyll a concentration |
[48] |
|
|
Eutrophic < 3
|
EAjk = June biomass of edible algae taxa metric score
|
[25] |
|
|
A lake trophic state evaluation index using the Rotifera community |
N = Rotifera numbers (ind./L)
|
[12] |
|
|
A lake trophic state evaluation index using the Crustacea community |
N = Numbers of Crustacea (ind./L)
|
[16] |
|
|
An index that measures the ecological water quality of a lake by combining the dry biomass of plankton
|
B = dry biomass(mg/L)
|
[26] |
|
|
bad ≤ 0.189
|
CA/CY = Ratio of Calanoida to Cyclopoida individual numbers(ind./L)
|
[43] |
|
|
Bad ≤ 6
|
AZoo = Abundance (ind./L)
|
[27] |
This entry is adapted from the peer-reviewed paper 10.3390/su151410950
References
- Allen, H.L. Primary productivity, chemo-organotrophy, and nutritional interactions of epiphytic algae and bacteria on macrophytes in the littoral of a lake. Ecol. Monogr. 1971, 41, 97–127.
- Welti, N.; Striebel, M.; Ulseth, A.J.; Cross, W.F.; DeVilbiss, S.; Glibert, P.M.; Guo, L.; Hirst, A.G.; Hood, J.; Kominoski, J.S.; et al. Bridging food webs, ecosystem metabolism, and biogeochemistry using ecological stoichiometry theory. Front. Microbiol. 2017, 8, 1298.
- Karr, J.R. Assessing Biological Integrity in Running Waters: A Method and Its Rationale; Illinois Natural History Survey Special Publication No. 05; Illinois Natural History Survey: Champaign, IL, USA, 1986.
- Jeppesen, E.; Noges, P.; Davidson, T.A.; Haberman, J.; Noges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297.
- Mehner, T.; Keeling, C.; Emmrich, M.; Holmgren, K.; Argillier, C.; Volta, P.; Winfield, I.J.; Brucet, S. Effects of fish predation on density and size spectra of prey fish communities in lakes. Can. J. Fish. Aquat. Sci. 2016, 73, 506–518.
- Park, K.S.; Shin, H.W. Studies on phyto-and-zooplankton composition and its relation to fish productivity in a west coast fish pond ecosystem. J. Environ. Biol. 2007, 28, 415.
- Bruce, L.C.; Hamilton, D.; Imberger, J.; Gal, G.; Gophen, M.; Zohary, T.; Hambright, K.D. A numerical simulation of the role of zooplankton in C, N and P cycling in Lake Kinneret, Israel. Ecol. Model. 2006, 193, 412–436.
- Korponai, J.; Braun, M.; Forró, L.; Gyulai, I.; Kövér, C.; Nédli, J.; Urák, I.; Buczkó, K. Taxonomic, functional and phylogenetic diversity: How subfossil cladocerans mirror contemporary community for ecosystem functioning: A comparative study in two oxbows= Sesgos en la diversidad taxonómica, funcional y filogenética de las comunidades vivas y subfósiles de cladóceros. Limnetica 2019, 38, 431–456.
- Wetzel, R.G.; Likens, G. Limnological Analyses; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000.
- May, L.; O’Hare, M. Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK. In Rotifera X: Rotifer Research: Trends, New Tools and Recent Advances, Proceedings of the Xth International Rotifer Symposium, held in Illmitz, Austria, 7–13 June 2003; Springer: Dordrecht, The Netherlands, 2005; pp. 397–404.
- Duggan, I.C.; Green, J.D.; Shiel, R.J. Distribution of rotifers in North Island, New Zealand, and their potential use as bioindicators of lake trophic state. In Rotifera IX: Proceedings of the IXth International Rotifer Symposium, Khon Kaen, Thailand, 16–23 January 2000; Springer: Dordrecht, The Netherlands, 2001; pp. 155–164.
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350.
- Ichise, S.; Wakabayashi, T. Illustrated Handbook of Freshwater Plankton in Japan; Godo Shuppan: Tokyo, Japan, 2005; pp. 116–117, 125.
- Dodson, S.I.; Frey, D.G. Chapter 22 Cladocera and other Branchiopoda. In Ecology and Classification of North American Freshwater-Invertebrates; Thorp, J.H., Covich, A.P., Eds.; Academic Press: San Diego, CA, USA, 2001.
- Lampert, W.; Sommer, U. Limnoecology: The Ecology of Lakes and Streams; Oxford University Press: Oxford, UK, 2007.
- Chen, G.; Dalton, C.; Taylor, D. Cladocera as indicators of trophic state in Irish lakes. J. Paleolimnol. 2010, 44, 465–481.
- Tumurtogoo, U.; Figler, A.; Korponai, J.; Sajtos, Z.; Grigorszky, I.; Berta, C.; Gyulai, I. Density and Diversity Differences of Contemporary and Subfossil Cladocera Assemblages: A Case Study in an Oxbow Lake. Water 2022, 14, 2149.
- Korponai, J.L.; Kövér, C.; López-Blanco, C.; Gyulai, I.; Forró, L.; Katalinic, A.; Ketola, M.; Nevalainen, L.; Lioto, T.P.; Sarmaja-Korjonen, K.; et al. Effect of temperature on the size of sedimentary remains of littoral chydorids. Water 2020, 12, 1309.
- Boxshall, G.A.; Defaye, D. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 2008, 595, 195–207.
- Perbiche-Neves, G.; Pomari, J.; Serafim-Júnior, M.; Nogueira, M.G. Cyclopoid copepods as indicators of trophic level in South American reservoirs: A new perspective at species level based on a wide spatial-temporal scale. Ecol. Indic. 2021, 127, 107744.
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 97, 16–35.
- Pinto-Coelho, R.; Pinel-Alloul, B.; Méthot, G.; Havens, K.E. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: Variation with trophic status. Can. J. Aquat. Sci. 2005, 62, 348–361.
- Sendacz, S.; Caleffi, S.; Santos-Soares, J. Zooplankton biomass of reservoirs in different trophic conditions in the State of São Paulo, Brazil. Braz. J. Biol. 2006, 66, 337–350.
- Van Egeren, S.J.; Dodson, S.I.; Torke, B.; Maxted, J.T. The relative significance of environmental and anthropogenic factors affecting zooplankton community structure in Southeast Wisconsin Till Plain lakes. Hydrobiologia 2011, 668, 137–146.
- Kane, D.D.; Gordon, S.I.; Munawar, M.; Charlton, M.N.; Culver, D.A. The Planktonic Index of Biotic Integrity (P-IBI): An approach for assessing lake ecosystem health. Ecol. Indic. 2009, 9, 1234–1247.
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Grazing potential—A functional plankton food web metric for ecological water quality assessment in Mediterranean lakes. Water 2019, 11, 1274.
- Stamou, G.; Mazaris, A.D.; Moustaka-Gouni, M.; Špoljar, M.; Ternjej, I.; Dražina, T.; Dorak, Z.; Michaloudi, E. Introducing a zooplanktonic index for assessing water quality of natural lakes in the Mediterranean region. Ecol. Inform. 2022, 69, 101616.
- Ejsmont-Karabin, J.; Karabin, A. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Pol. J. Ecol. 2013, 61, 561–573.
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 1975, 19, 75–97.
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. BioScience 1985, 35, 634–639.
- Wickham, S.A. The direct and indirect impact of Daphnia and Cyclops on a freshwater microbial food web. J. Plankton Res. 1998, 20, 739–755.
- Perga, M.E.; Kainz, M.; Matthews, B.; Mazumder, A. Carbon pathways to zooplankton: Insights from the combined use of stable isotope and fatty acid biomarkers. Freshw. Biol. 2006, 51, 2041–2051.
- Khan, Q.; Khan, M. Effect of temperature on waterflea Daphnia magna (Crustacea: Cladocera). Nat. Preced. 2008, 1.
- Korponai, J.; Magyari, E.K.; Buczkó, K.; Iepure, S.; Namiotko, T.; Czakó, D.; Kövér, C.; Braun, M. Cladocera response to Late Glacial to Early Holocene climate change in a South Carpathian mountain lake. Hydrobiologia 2011, 676, 223–235.
- Ekvall, M.K.; Urrutia-Cordero, P.; Hansson, L.A. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS ONE 2014, 9, e112956.
- Berta, C.; Tóthmérész, B.; Wojewódka, M.; Augustyniuk, O.; Korponai, J.; Bertalan-Balázs, B.; Nagy, A.S.; Grigorszky, I.; Gyulai, I. Community response of Cladocera to trophic stress by biomanipulation in a shallow oxbow lake. Water 2019, 11, 929.
- Duggan, I.C.; Green, J.D.; Shiel, R.J. Distribution of rotifer assemblages in North Island, New Zealand, lakes: Relationships to environmental and historical factors. Freshw. Biol. 2002, 47, 195–206.
- Jeppesen, E.; Peder Jensen, J.; SØndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218.
- Tessier, A.J.; Horwitz, R.J. Influence of water chemistry on size structure of zooplankton assemblages. Can. J. Aquat. Sci. 1990, 47, 1937–1943.
- Stemberger, R.S.; Lazorchak, J.M. Zooplankton assemblage responses to disturbance gradients. Can. J. Aquat. Sci. 1994, 51, 2435–2447.
- Beaver, J.R.; Tausz, C.E.; Renicker, T.R.; Holdren, G.C.; Hosler, D.M.; Manis, E.E.; Scotese, K.C.; Teacher, C.E.; Vitanye, B.T.; Davidson, R.M. The late summer crustacean zooplankton in western U.S.A reservoirs reflects ecoregion, temperature and latitude. Freshw. Biol. 2014, 59, 1173–1186.
- Du, X.; García-Berthou, E.; Wang, Q.; Liu, J.; Zhang, T.; Li, Z. Analyzing the importance of top-down and bottom-up controls in food webs of Chinese lakes through structural equation modeling. Aquat. Ecol. 2015, 49, 199–210.
- Ochocka, A. ZIPLAS: Zooplankton Index for Polish Lakes’ Assessment: A new method to assess the ecological status of stratified lakes. Environ. Monit. Assess. 2021, 193, 664.
- Mäemets, A. Izmenenija zooplanktona. In Antropogennoe Vozdeistvije na Malye Ozera ; Nauka: Leningrad, Russia, 1980; pp. 54–64.
- Barbour, M.T.; Stribling, J.B.; Karr, J.R. Multimetric approach for establishing biocriteria. Biological assessment and criteria. In Tools for Water Resource Planning and Decision Making; CRC Press: Boca Raton, FL, USA, 1995; pp. 63–77.
- Hering, D.; Feld, C.K.; Moog, O.; Ofenböck, T. Cook book for the development of a Multimetric Index for biological condition of aquatic ecosystems: Experiences from the European AQEM and STAR projects and related initiatives. In The Ecological Status of European Rivers: Evaluation and Intercalibration of Assessment Methods; Springer: Dordrecht, Switzerland, 2006; pp. 311–324.
- Chen, K.; Hughes, R.M.; Brito, J.G.; Leal, C.G.; Leitao, R.P.; de Oliveira-Junior, J.M.B.; de Oliveira, V.C.; Dias-Silva, K.; Ferraz, S.F.B.; Ferreira, J.; et al. A multi-assemblage, multi-metric biological condition index for eastern Amazonia streams. Ecol. Indic. 2017, 78, 48–61.
- Moss, B.; Stephen, D.; Alvarez, C.; Becares, E.; Bund, W.V.D.; Collings, S.E.; Donk, E.V.; Eyto, E.D.; Feldmann, T.; Fernández-Aláez, C.; et al. The determination of ecological status in shallow lakes—A tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquat. Conserv. Mar. Freshw. 2003, 13, 507–549.
- Kairesalo, T.; Tátrai, I.; Luokkanen, E. Impacts of waterweed (Elodea canadensis Michx) on fish-plankton interactions in the lake littoral. Int. Ver. Für Theor. Und Angew. Limnol. Verhandlungen 1998, 26, 1846–1851.
- Mack, H.R.; Conroy, J.D.; Blocksom, K.A.; Stein, R.A.; Ludsin, S.A. A comparative analysis of zooplankton field collection and sample enumeration methods. Limnol. Oceanogr. Methods 2012, 10, 41–53.
- Hillbricht-Ilkowska, A. Morphological variation of Keratella cochlearis (Gosse)(Rotatoria) in several Masurian lakes of different trophic level. Pol. Arch. Hydrobiol. 1972, 19, 253–264.
- Pejler, B.I.R.G.E.R. Taxonomic notes on some planktic Rotifers. Zool. Bidr. Från Upps. 1962, 35, 307–319.
- Andronikova, I.N. Zooplankton characteristics in monitoring of Lake Ladoga. Hydrobiologia 1996, 322, 173–179.
- Caroni, R.; Irvine, K. The potential of zooplankton communities for ecological assessment of lakes: Redundant concept or political oversight? In Biology and Environment: Proceedings of the Royal Irish Academy; Royal Irish Academy: Dublin, Ireland, 2010; Volume 110, pp. 35–53. Available online: https://www.muse.jhu.edu/article/809710 (accessed on 9 July 2023).
- Haberman, J.; Haldna, M. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Vőrtsjärv. J. Limnol. 2014, 73, 263–273.
This entry is offline, you can click here to edit this entry!