Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As a cellulose-based polymer, the combustion of cotton is an exothermic oxidation process that takes place upon heating, consuming flammable gases, liquids, and solid residues produced during the pyrolysis of the textile material, thus generating heat.
- inorganic flame retardants
- sol–gel technology
- nanoclays
- functional nanofillers
1. Introduction
In the last decades, continuous improvements in people’s living standards, together with environmental problems and population growth, have generated a demand for advanced materials, thus resulting in ever-accelerating scientific progress and research. In this regard, a special place is covered by textiles, which are present in everyday human life, not only for conventional clothes and accessories, but also as decoration and comfort elements in homes, and public and private buildings, as components in transportation, and as structural elements for buildings. As a matter of fact, besides the conventional textiles sector, more complex fabric manufacturing is growing, aiming to a high level of product innovation and cutting-edge process technologies, continuously searching for specific textiles aesthetics and high-quality characteristics, as well as new functionalities (i.e., resulting from hybrid material additives capable of providing protection, comfort, and performance to the final textile products). At this stage, it appears very clear that textile fabrics must be treated not only as artistic surfaces for fashion but as materials between the wearers and the surroundings for all intents and purposes, with their tunable intrinsic structures and performances.
Today, the so-called technical textiles, designed for their functional properties rather than their aesthetic features, have become a revolutionary product category, providing the foundation for an entirely new variety of applications [1]. They can be utilized for various industrial sectors, also thanks to the possibility of introducing many functionalizations during the finishing step, such as water-repellent, antibacterial, or flame-retardant properties, among others [1,2]. Keeping an eye on nature and human health protection, stringent environmental restrictions and greater interest in natural resource usage have recently led to an increasing trend toward the exploitation of natural fibers in technological applications as eco-friendly options to synthetic counterparts [3].
Among others, cotton fabric represents one of the most widely employed natural fibers in the textile industry. Indeed, the average global production of cotton fibers reached about 25 million tonnes during recent years, representing 28% of all fibers’ global production [4]. Cotton fiber structure consists of a polysaccharide component of β-d-glucopyranose units linked together by β-1,4 bonds (Figure 1); non-reducing and reducing sugar units stabilize the end terminal of cellulose polymer chains [5]. Chemical modifications and fiber behavior are mainly attributable to the -OH groups on positions C-2, C-3, and C-6 of the D-glucopyranosyl units [5,6].
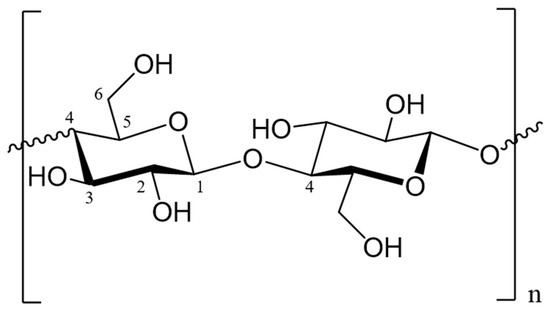
Figure 1. Cellulose structure with carbon atoms numbering. Numbers (1 and 4) were used in the text to explain the link between monomers (Cotton fiber structure consists of a polysaccharide component of β-d-glucopyranose units linked together by β-1,4 bonds).
Due to these superior characteristics (i.e., fast moisture absorption, mechanical properties, breathability, softness, comfort, biodegradability, and good thermal conductivity), cotton is used extensively in clothing, bedding, furniture, and wall hangings, as well as in apparel manufacturing, home furnishings, medical textiles, and other industrial products [7]. Moreover, cotton-based textiles have been used as protective clothing for workers and, more generally, as uniforms employed in workplaces where there is a chance of accidental contact with flames [8]. However, its disadvantage is represented by the easy ignition and aptitude to burn in the air (its limiting oxygen index, LOI, is around 18%) [9] as well as the quick flammability propensity, which have restricted its application in particular fields requiring textiles with enhanced flame retardancy [10]. As a significant component in a range of everyday textiles, most of the research efforts have been addressed to investigate the flammability of cotton fabrics [11].
Currently, the research on flame-retardant treatments for fabrics has become a necessary pathway, and many countries have created relevant testing standards and legislation to standardize the market [12]. As reported in the scientific literature [13], the estimated costs from fire losses are approximately 1% of the global gross domestic product. Only in the United States, home fires are the most frequent type of fire accidents [14]: the single most significant cause of civilian deaths refers to home fires (about 24%), due to the presence of residential upholstered furniture, with a yearly estimated average of 8900 fires, 610 deaths, and 1120 injuries, resulting in $566 million in direct damage [15]. Furthermore, the annual United Kingdom fire statistics demonstrate that most of the fire accidents that occur in houses involve upholstering furniture, bedding, and nightwear [16]. According to statistics from the International Association of Fire and Rescue Services [17], in the period 2016–2020, several fires involving textiles as a major fuel element have driven the research toward the development of new fire precautionary and preventative procedures.
The most common flame-retardant (hereafter, FR) finishes for cotton fabrics were developed from 1950 to 1980, and were based on halogen derivatives, phosphorous, and/or nitrogen [18]. In particular, during the combustion of halogen-containing flame-retardant fabrics, toxic gases, such as hydrogen halide, are produced, causing harm to human health and environmental pollution. Because of these concerns, halogenated finishes have been rigorously restricted in textile finishing [19].
In this regard, the development of flame-retardant textile finishing able to provide self-extinguishing properties and delay or inhibit the flame spread is relevant. Accordingly, the flame-retardant effect is attained when at least one or more factors among fuel, heat, and oxygen are reduced or eliminated, thus further enhancing the thermal stability of the polymer fabric and quenching the formed high-energy free radicals. Generally, flame-retardant mechanisms involve chemical, physical processes, or a combination of both. Mainly, the action mechanism of flame retardants can be traced back as follows [20]:
-
Gas phase. FRs following this mechanism act by diluting the gas phase and/or by chemical quenching of active radicals. The former effect is based on releasing non-combustible gases (e.g., H2O and CO2) that can dilute the oxygen or the fuel concentrations by lowering them under the flammability limit. Metal hydroxides and carbonates are generally believed to follow this mode of action due to their endothermic thermal decomposition and production of non-combustible gases. On the other side, because of the occurrence of radical reactions during combustion, the flame retardants decompose in radical species able to quench the high-energy free radicals formed during cellulose combustion (e.g., H• and •OH) by decreasing the burning rate of the combustible materials and, finally, interrupting the exothermic reactions of the combustion. However, the mechanism in the gas phase may be slightly different, depending on the used chemicals [21];
-
Condensed phase. The thermal cracking reaction process of cellulose can be modified by flame retardants. Indeed, many reactions (e.g., dehydration, condensation, cross-linking, and cyclization) take place at lower temperatures by producing coherent carbon layers on the fabric surface, thus lowering both the evolution of combustible gases and the decomposition rate of the fabric. For more in detail, the depolymerization of fiber materials is observable under the action of the flame retardant, as well as a decrease in the melting temperature that leads to a higher temperature difference between the melting and ignition point [22]. According to the chemical structure of the employed FR, an intumescent effect can also be observable. Moreover, a certain amount of heat is absorbed by interrupting the feedback of the heat to the fibers and, finally, the combustion process. Inorganic finishes containing phosphorus, boron, sol–gel precursors, nanoclay, and metal-based finishes, as well as carbon nanotubes and graphene, are believed to follow this mode of action.
However, combustion is a complex process, and the action of flame retardants may occur across both gas and condensed phases or in just one of the two. Indeed, synergistic flame-retardant actions can be obtained by combining different mechanisms that, conversely, are barely assignable to a single flame-retardant system [23].
As an alternative to most common FR treatments, whose flame-retardant mechanism mainly occurs in the gas phase, formulations acting in the condensed phase and containing nitrogen and phosphorus have been developed, promoting the formation of a char during thermal degradation, which provides an insulating layer to the underlying polymer. Indeed, organophosphate flame retardants containing synergistically active nitrogen may be more effective than pure phosphate flame retardants.
2. Mechanism of Cotton Combustion
As a cellulose-based polymer, the combustion of cotton is an exothermic oxidation process that takes place upon heating, consuming flammable gases, liquids, and solid residues produced during the pyrolysis of the textile material, thus generating heat. The process is considered to involve mainly four stages: heating, pyrolysis, ignition, and flame spread. During the heating step, the temperature of the cotton is raised by external ignition to a level that depends on the intensity of the ignition source and the thermal properties of the textile. When cellulose is heated at temperatures not exceeding 150 °C, water desorption occurs.
Once the cellulosic material exceeds 250 °C, pyrolysis occurs as an endothermic process [35,36], resulting in the irreversible polymer decomposition by producing tar (of which levoglucosan is the main component), flammable gases (methane, ethane, and carbon monoxide), non-flammable gases (carbon dioxide and formaldehyde), other by-products (water, alcohols, organic acids, aldehydes, and ketones), and char. If the combustion process generates sufficient heat, the heat is further transferred to the textile substrate, further accelerating the degradation processes and leading to a “self-sustaining” combustion cycle (Figure 2). The thermal decomposition of cotton fabrics generates approximately 51% water and gases, 47% tar, and 2% char [37].
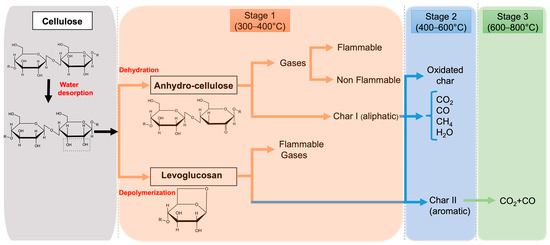
Figure 2. Schematic process of thermal degradation of cellulose.
More specifically, the depolymerization of cellulose can be mainly divided into three stages as a function of the temperature range:
- -
-
a first stage (between 300 and 400 °C), corresponding to the pyrolysis that takes place through two competing decomposition reactions: dehydration and depolymerization [38]. The former produces anhydro-cellulose (dehydro-cellulose), which further decomposes at higher temperatures and produces various volatile products such as alcohols, alkanes, aldehydes, fuel gases, carbon monoxide, methane, ethylene, and non-flammable gases (carbon dioxide and water vapor), and an aliphatic char (char I). Otherwise, depolymerization by breaking of glycosidic linkages results in the formation of tar (condensed phase), which is mostly composed of levoglucosan and glycolaldehyde (hydrogen acetaldehyde);
- -
-
a second stage (between 400 and 600 °C), corresponding to the competitive conversion of aliphatic char to aromatic and char oxidation [38]. In this stage, tar is further decomposed into small volatile flammable molecules and aromatic char (char II, stable up to 800 °C), while volatile compounds from Stage 1 are also oxidized to produce similar oxidized char and aromatic molecules;
- -
The heating rate has been proven to influence the concentration of fuel products during the overall combustion steps: in particular, low heating rates promote dehydration and subsequent char formation, whereas high heating rates lead to depolymerization and fast volatilization by forming levoglucosan (whose yield can be reduced by dehydration, thus resulting in the formation of fewer volatile species), as well as higher gaseous flammable products. The formed char acts as an insulating layer on the surface of the cellulosic material, reducing the heat transfer, and as a diffusion barrier for combustible gases. Moreover, the level of material protection is strictly affected by its structure: a char, featuring an open channel structure, promotes the transfer of combustible gases to the flame, while a char with a closed structure hinders volatile species to reach the flame. Furthermore, the formation of char leads to condensed water that dilutes the flammable gases [17].
Generally, neat cellulose fibers lead to the formation of around 13% char, an amount that can be significantly enhanced by using FRs, thus reaching 30–60% [43]. Moreover, the presence of FRs on textile surfaces slows down the combustion due to the low heat involved, which is required to maintain pyrolysis. As a result of the highly flammable nature of cellulose-based materials, as a consequence of their chemical composition, a fire-hindering mechanism integrated into textiles is needed to decrease or eliminate possible fire dangers.
This entry is adapted from the peer-reviewed paper 10.3390/inorganics11070306
This entry is offline, you can click here to edit this entry!
