Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cyclic peptides are molecules that are already used as drugs in therapies approved for various pharmacological activities, for example, as antibiotics, antifungals, anticancer, and immunosuppressants. Interest in these molecules has been growing due to the improved pharmacokinetic and pharmacodynamic properties of the cyclic structure over linear peptides and by the evolution of chemical synthesis, computational, and in vitro methods.
- bioactivity
- clinical trials
- cyclic peptides
- cyclization
- pipeline
1. Introduction
Peptides, molecules that contain two or more residues of amino acids linked by an amide bond [1], can be considered to fall between small molecules and large biological molecules, such as proteins or antibodies [2]. Peptides have several advantages over small molecules used in conventional therapy, such as high selectivity, potency, biotarget specificity, few side effects, and low accumulation in tissues [3][4]. When compared to proteins and antibodies, peptides have the advantage of a lower immunogenicity [2].
Over the years, the therapeutic potential of peptides has been exploited for a broad spectrum of biological activities, such as antimicrobial, antihypertensive, antioxidant, anticancer, antidiabetic, and anti-inflammatory, among others, which attract the attention of the pharmaceutical [5][6][7], cosmeceutical [8], and nutraceutical [9][10] industries.
The era of therapeutic peptides began with the first medical administration of insulin in the 1920s [11]. This discovery revolutionized the treatment of patients with type I diabetes, and peptides were seen as potential therapeutic tools [12]. About 40 years later, the first hormones used in clinical practice, oxytocin, and vasopressin, were synthesized [13]. Some industrial groups have dived into this field, and the interest in this type of molecules increased [14]. While the advantages of using these molecules were explored, their limitations also emerged at a time when the discovery and development of small molecules was at its peak. This circumstance has led to the stagnation of research on peptides as drugs. Despite this, peptides continued to be explored as tools for the study of targets, and in the 1980s, the interest in these molecules returned backed by biotechnology companies [14]. Since then, the tendency of approved peptides as therapeutic agents has been increasing [5].
In addition to their therapeutic potential, peptides can also be used in imaging and disease diagnosis [5]. For example, it is known that peptides composed of arginine-glycine-aspartate (RGD) moiety have an affinity to bind to integrins, which are heterodimeric receptors that play pivotal roles in cells. By binding to integrin, RGD peptides can prevent angiogenesis, a process involved in diseases, such as cancer and rheumatoid arthritis [15][16][17]. These peptides can also be used for tumor imaging when linked to radioisotopes or even to create tumor-targeted drug delivery systems reducing the adverse effects inherent to conventional chemotherapy [18][19].
Until May 2023, one hundred and fourteen peptides have been approved by the regulatory authorities as therapeutic agents (Figure 1), which included pharmaceuticals and diagnostic tools [20].
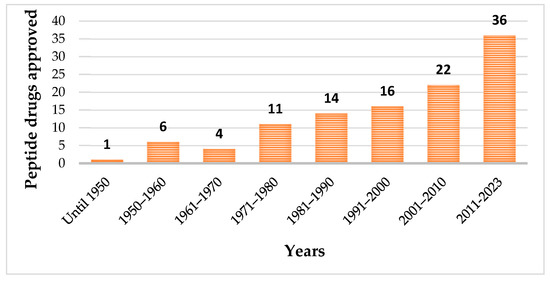
Figure 1. Evolution of approved peptide drugs over the years.
Among the approved peptides, the cyclic peptides represent 46% of the total approvals (Figure 2A) [21]. Gramicidin S (antibiotic) was the first cyclic peptide to be used as a drug. Its discovery in 1944 by Gause and Brazhnikova during the Second World War and its use in Soviet military hospitals revolutionized the field of cyclic peptides [22][23]. Other interesting examples are telavancin, dalbavancin, and oritavancin (semi-synthetic cyclic lipoglycopeptide antibiotics) [24], anidulafungin (from the class of echinocandin antifungals) [25], lanreotide, pasireotide, and romidepsin (anticancer drugs), and linaclotide (derived from an enterotoxin for gastrointestinal (GI) disorders) [26]. The last approved cyclic peptide was rezafungin (antifungal analog of anidulafungin), being approved by Food and Drug Administration (FDA) in 2023 [27]. This drug is administered orally and has a half-life of 30 h, which represents an advance in pharmacokinetic characteristics in comparison with other peptide drugs. In fact, regarding the routes of administration of approved peptides (Figure 2B), parenteral administration is the most frequent, of which the intravenous route is the most recurrent [21].
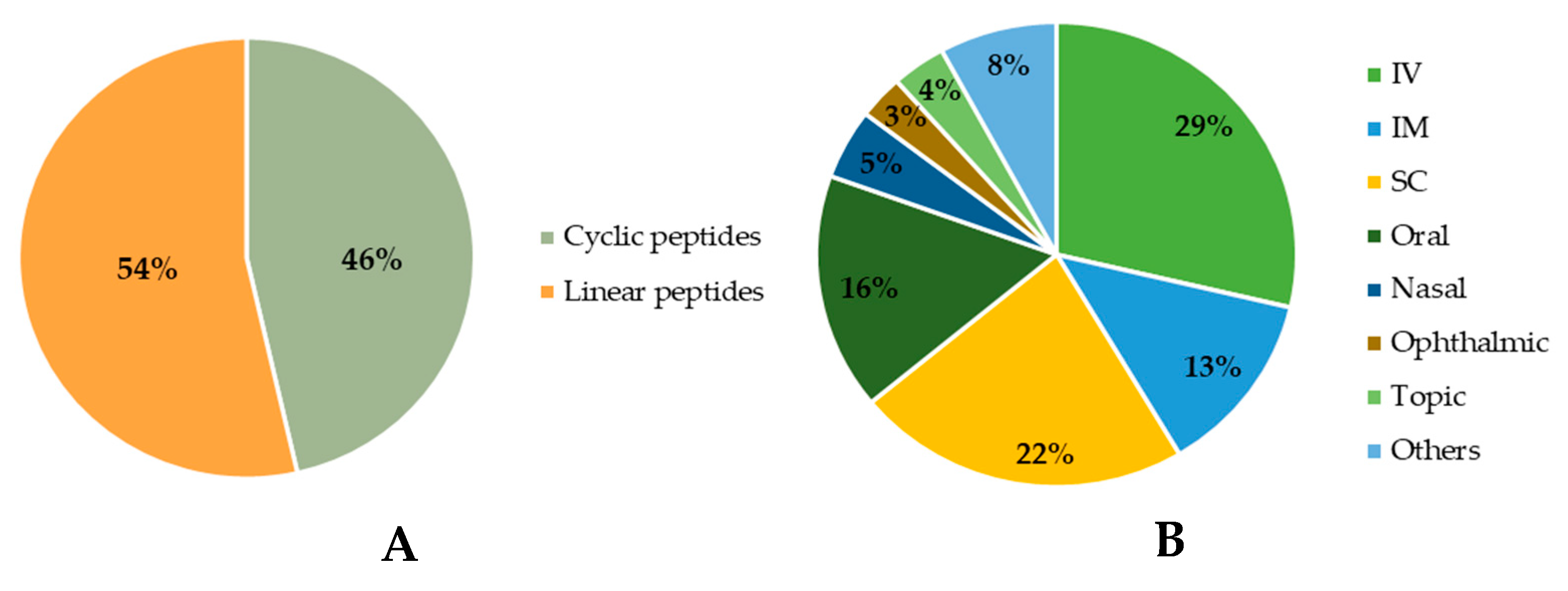
Figure 2. Comparison between approved cyclic and linear peptides (A). Routes of administration of approved peptides (B). IV: Intravenous; IM: Intramuscular; SC: Subcutaneous.
Another relevant application of peptides is in drug delivery, considering the good hydrophilicity/hydrophobicity ratio, as well as intra and intermolecular interaction of amino acids by weak non-covalent bonds, which makes them capable of organizing themselves to form nanostructures. Peptide nanostructures have demonstrated a great drug load capacity and drug protection and are responsive to external stimuli [28]. Recently, new cyclic peptides exhibiting nanospherical structures demonstrated the ability to form stable complexes with short-interfering RNA (siRNA), proving to be a promising tool in nucleic acid delivery for cancer treatment, as an example [29].
Moreover, cyclic peptides, such as vancomycin, teicoplanin, and ristocetin (macrocyclic antibiotics), in addition to their therapeutic actions, were also explored as chiral stationary phases for chromatographic applications [30][31]. This application of macrocyclic antibiotics in liquid chromatography was introduced by Armstrong et al. in 1994 [32]. The high number of stereogenic centers and the macrocyclic structure of these peptides allow a variety of interactions with the analytes to enantioseparate and the possibility of forming inclusion complexes, which contribute to their high capacity of chiral recognition [33][34].
Cyclic peptides can be obtained from natural sources, both terrestrial and marine [35][36]. With regard to terrestrial sources, these can be from animal origin [37], such as venoms [38] (a rich source of bioactive peptides revised in [39]), plants [40], microorganisms [41], among others). Examples of peptides obtained from terrestrial sources are the antibiotics vancomycin (isolated from the soil bacterium Amycolatopsis orientalis) [42], daptomycin (from the soil bacterium Streptomyces roseoporus) [43], teixobactin (from the soil bacterium Eleftheria terrae) [44], and apamin (isolated from bee Apis mellifera) [45].
Bioactive marine cyclic peptides can be found in marine tunicates [46][47], sponges [47][48], algae [49], bacteria [50][51], cyanobacteria [52][53], fungi [54][55], and other invertebrates [56], including symbionts [57] and non-symbiotic microorganisms, such as sponge-associated fungi [58][59]. Marine-derived peptides display a broad spectrum of bioactivities [60][61], mainly anticancer [62] and antimicrobial [63] being one of the research topics that gives a very high output, with a considerable increase in the number of publications (268 per year), from 2010 to 2020 [64]. To highlight that, one marine cyclic peptide-derived drug has reached the market—ziconotide [65] (the first FDA-approved marine peptide, in 2004).
Chemical synthesis is also a remarkable source of peptides, which allows obtaining an appropriate amount of compound to carry out further large-scale biological assays, including studies of the mechanism of action, pharmacokinetics, toxicity, and others [66][67][68][69]. In the literature, several reports can be found describing synthetic routes for peptides, including very large and complex structures [70][71]. In addition, the synthesis allows for obtaining the structurally diverse analogs and derivatives of the natural peptides with improved properties for structure–activity relationship (SAR) studies [72]. The synthetic strategies for molecular modifications can include the following: (1) incorporation of non-proteinogenic amino acids to prevent proteolysis of peptides [73]; (2) acetylation of the N-terminus of short peptides to increase peptidase stability in serum and, consequently, enhance the half-life [74]; (3) glycosylation to improve protein–protein interaction, protein permeability, metabolic stability, and bioavailability [75]; lipidation to enable the binding to a carrier serum protein and, consequently, enhance the half-life, among others [76][77].
Typically, there are two strategies to synthesize peptides: solution-phase and solid-phase peptide synthesis (SPPS) [63]. They include two key steps in the formation of a peptide bond between two amino acids: the activation of the carboxyl group by coupling agents and the use of temporary protecting groups to direct the reaction to the desired direction [78]. The two most used strategies are fluorenylmethyloxycarbonyl (Fmoc)/tert-butyl (tBu) and tert-butyloxycarbonyl (Boc)/benzyl (Bn) strategies [79]. In addition to classical peptide synthesis techniques [69], significant efforts have been carried out for the introduction of sustainable and innovative processes for synthesis and purification methodologies [80][81].
Despite several advantages of peptides, they also have characteristics that are disadvantageous when they are used for drug development, mainly concerning pharmacokinetic issues: (1) they have a short half-life in the plasma because of the action of peptidases; (2) are easily degraded by enzyme actions and pH hydrolysis on the GI tract, which makes them not bioavailable orally; (3) have low membrane permeability, which makes its passage through membranes on absorption locals and intracellular biotargets difficult [2]. Nevertheless, the pharmacokinetic properties of these molecules can be improved with some strategies of molecular modifications, such as conjugation with polyethylene glycol (PEG), albumin, or proteins, as well as other approaches, such as cyclization [14].
Cyclization of peptides has proven to be an asset in enhancing the advantages of linear peptides, but also a way to overcome their disadvantages. Cyclization of a peptide reduces the spatial vibrations of the molecule leading to a decrease in conformational changes. In addition, cyclization induces an increase in the surface area available for interaction with the biological target. These two reasons lead to an increase in binding affinity and selectivity to the target [4]. In addition to pharmacodynamic considerations, the pharmacokinetic properties of peptides can also be improved by cyclization, as the rigidification of the structure leads to a lowering of the energy barrier required for the peptide to adapt to the membrane environment and bind to transport proteins to enter the cell by passive diffusion or active transport. Thus, cyclization can improve the absorption and membrane permeability of peptides [5]. Cyclization of peptides also gives greater metabolic stability, as cyclic peptides are resistant to the action of exopeptidases, due to the lack of terminal amine and carboxylic acid groups, and endopeptidases, by blocking the access to the cleavage site [19]. Although cyclization may improve the pharmacokinetic properties of peptides, it is important to highlight that for many cyclic peptides, the poor pharmacokinetic parameters are one of the main reasons for failure in phase I/II trials [82]. Through chemical synthesis, it is possible to carry out molecular modifications to obtain derivatives with improved characteristics [14].
2. Cyclic Peptides in the Pipeline
Currently, there are various cyclic peptides in clinical development to cover such diverse medical conditions as cancer, infectious diseases, and hematological disorders, among others. Table 1 summarizes 27 cyclic peptides in clinical trials, including their generic names, therapeutic indication(s), current stage of development, name of the pharmaceutical company responsible for the development, and source (natural or synthetic).
Table 1. Cyclic peptides currently in clinical trials.
| Peptide Name | Generic Name | Indication | Highest Phase | Company | Source | References |
|---|---|---|---|---|---|---|
| POL6326 | Balixafortide | Advanced breast cancers | Spexis | Synthesis | [83] | |
| BL-8040 | Motixafortide | HSCs, solid tumors, and AML | III | Bioline RX | Synthesis | [84] |
| BT1718 |  |
Cancer with MT1-MMP expression | I/II | Bicycle Therapeutics Ltd. |
Synthesis | [85][86][87] |
| BT8009 |  |
Cancer types, where Nectin-4 is expressed | I/II | Bicycle Therapeutics Ltd. |
Synthesis | [88][89] |
| BT5528 |  |
Advanced solid tumors associated with EphA2 expression | I/II | Bicycle Therapeutics Ltd. |
Synthesis | [90] |
| VT1021 |  |
Antitumor | II | Vigeo Therapeutics |  |
[91] |
| ALRN-6924 |  |
Chemoprotective agent | Ib | Aileron Therapeutics | Synthesis | [92] |
| CEND-1 |  |
Enhance the efficacy of chemotherapy | II | Cend Therapeutics |  |
[93] |
| POL7080 | Inhaled murepavadin | Antibiotic to treat Pseudomonas infections in patients with cystic fibrosis | I | Spexis | Synthesis | [94] |
 |
Thanatin-derivative |  |
Pre-clinical | Spexis/University of Zurich |  |
[95] |
| CD-101 | Rezafungin | Treatment of candidemia and/or invasive candidiasis and invasive fungal diseases |
III | Cidara Therapeutics Inc. | Semi-synthesis | [96] |
| RA-101495 | Zilucoplan | Paroxysmal nocturnal hemoglobinuria, generalized myasthenia gravis | III | UCB Pharma | Synthesis | [97] |
| AP301 | Solnatide | Pulmonary permeability edema | II | Apeptico | Synthesis | [98] |
| POL6014 | Lonodelestat | Cystic fibrosis | II | Santhera Pharmaceuticals | Synthesis | [99] |
| THR-149 | Diabetic macular edema | II | Oxurion NV | Synthesis | [100][101] | |
| PTG-300 | Rusfertide | Polycythemia vera | III | Protagonist Therapeutics, Inc. | Synthesis | [102][103] |
| PN-943 |  |
Ulcerative colitis | II | Protagonist Therapeutics, Inc. |  |
[104][105] |
| PL8177 |  |
Ulcerative colitis | II | Palatin Technologies Inc. |
Synthesis | [106] |
| PA-001 |  |
SARSCov-2 infection | I | PeptiAID |  |
[107] |
| AZP-3813 |  |
Acromegaly | IND-Enabling | Amolyt Pharma |  |
[108] |
| PL9643 |  |
Dry eye disease | III | Palatin Technologies Inc. |
Synthesis | [109] |
| PM90001 | Plitidepsin | SARS-CoV-2 infection | III | PharmaMar | Synthesis | [46][110] |
| APL-2 | Pegcetacoplan | ALS, IC-MPGN, and C3G, CAD, and HSCT-TMA |  |
Apellis Pharmaceuticals Inc. |  |
[111] |
 |
Friulimicin B | Antibacterial activity against Gram-positive bacteria | Stopped in phase I in 2008 due to unfavourable pharmacokinetic properties | MerLion Pharmaceuticals GmbH | Nature | [112][113] |
| CB-183,315 | Surotomycin | Antibacterial activity against Clostridium difficile | Stopped in phase III in 2019 (no improvement over vancomycin) | Merck |  |
[114] |
| ShK-186 | Dalazatide | Plaque psoriasis | I (finished in May 2015) | Kv1.3 | Synthesis | [115] |
 |
Ularitide | Acutely decompensated heart failure | III (finished in October 2018) | Cardiorentis | Synthesis | [116][117] |
HSCs—hematopoietic stem cells; AML—acute myeloid leukemia; MT1-MMP—membrane type I matrix metalloproteinase; ALS—amyotrophic lateral sclerosis; IC-MPGN and C3G—immune complex membranoproliferative glomerulonephritis and C3 glomerulopathy; CAD—cold agglutinin disease; HSCT-TMA—hematopoietic stem cell transplant-associated thrombotic microangiopathy.
The 21 new cyclic peptides that are being used in clinical trials for the first time for a particular disease or condition are described here. The reported structures of these molecules are illustrated in Figure 3, and their development and clinical aspects are detailed in the next sections.
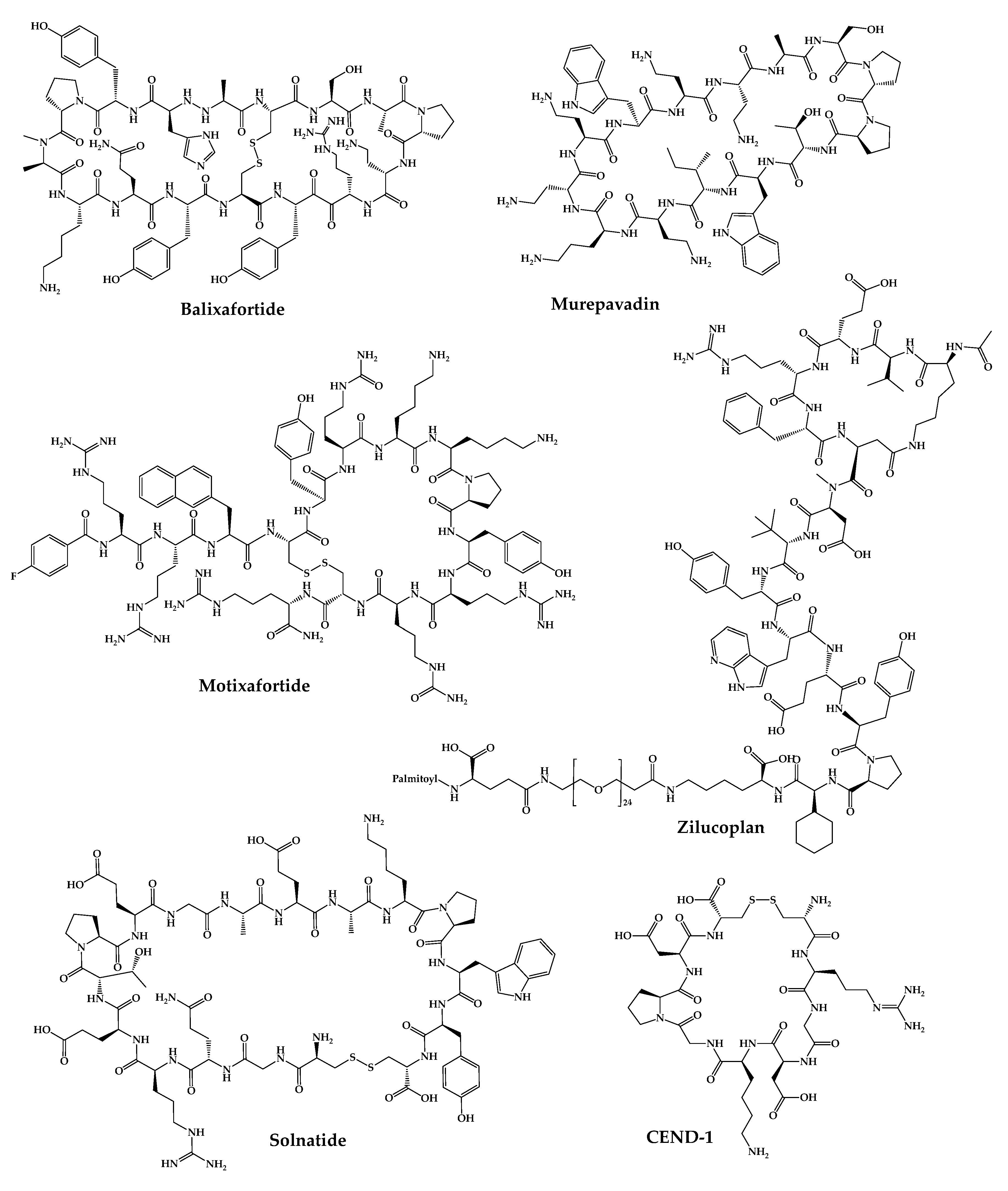
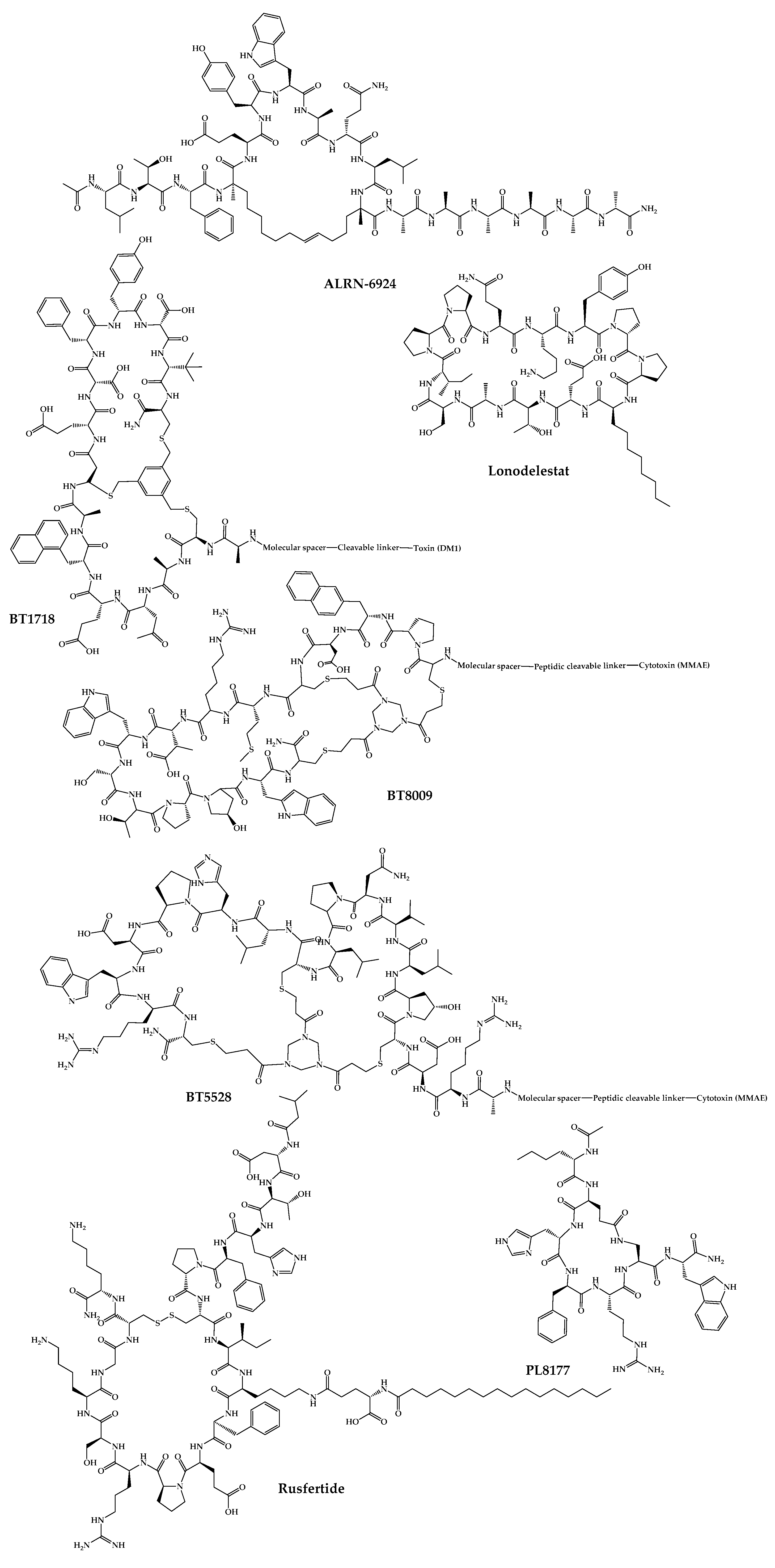
Figure 3. Available structures of the cyclic peptides currently in clinical trials as new chemical entities.
2.1. POL6326—Balixafortide
Balixafortide (POL6326) is a cyclic synthetic peptide with 14 amino acids developed by Spexis. It is a very potent and selective antagonist of chemokine receptor C-X-C chemokine receptor type 4 (CXCR4), inhibiting tumor growth and metastasis, as well as activating the immune response in the tumor microenvironment [118][119]. Balixafortide was developed by using a protein epitope mimetic (PEM) technology created by Polyphor (now Spexis) to discover and optimize fully synthetic CXCR4 inhibitor cyclic peptides [120]. The development process of cyclic peptides followed a rational approach since the structures mimic the natural β-hairpin loop of the target protein combined with phage display as a tool to find new hits for further modification and optimization [121].
POL6326 has a half-life of 6 to 8 h and demonstrated high potency and selectivity in preclinical trials [122]. In December 2018, a phase I clinical trial began to assess the efficacy, safety, and tolerability of balixafortide administered with eribulin versus eribulin alone in the treatment of human epidermal growth factor receptor type 2 (HER2) negative, locally recurrent or metastatic breast cancer (ClinicalTrials.gov identifier: NCT01837095) [122][123]. In the same month, a phase III clinical trial began (ClinicalTrials.gov identifier: NCT03786094) [124]. Nevertheless, in August 2021, the end of this study was announced because it was found that the administration of balixafortide together with eribulin does not significantly increase the activity of eribulin alone; therefore, the initial hypothesis of this study failed [125]. Recently, in July 2022, Spexis revealed that balixafortide demonstrated to be more effective when given together with docetaxel, when compared with their administration alone, in a preclinical model of prostate cancer [126]. Additionally, the potential of this cyclic peptide is also being explored for applications in solid and hematological tumors, as well as in rare non-oncological diseases [126]. Furthermore, balixafortide has already shown promising results in vitro, exhibiting cell protection in a SARS-CoV-2-induced cytopathic effect assay with a half maximal effective concentration (EC50) of 10 µM. These promising results have also been confirmed in vivo, where the amount of virus in hamster blood after SARS-CoV-2 infection was significantly reduced [83]. These preliminary results suggested that it may constitute a new treatment for COVID-19.
2.2. BL-8040—Motixafortide
Motixafortide (BL-8040) is a selective inhibitor of the CXCR4 receptor developed by BiolineRx. This peptide has a high binding affinity (IC50 ≈ 1 nM) and long receptor occupancy when compared to other inhibitors of this receptor [127][128].
Motixafortide is a synthetic cyclic peptide with 14 amino acids whose development began with molecular modifications of a natural defense peptide, polyphemusin. Applying this strategy, three inhibitory analogs of the CXCR4 receptor with activity against human immunodeficiency virus (HIV) were obtained [129][130]. Among all the synthesized analogs, T140 showed the highest inhibitory activity against the entry of HIV-1 and the highest binding affinity to the CXCR4 receptor [131]. T140 peptide was synthesized by SPPS using Fmoc as a protecting group followed by the one-pot technique where trimethylsilyl chloride—dimethyl sulfoxide/trifluoroacetic acid (TFA) were used simultaneously to cleave the peptide from the resin, deprotect the protecting groups and form the disulfide bond [131]. Since the T140 peptide had low serum stability, some analogs were synthesized, including motixafortide [129].
This peptide is currently being developed for two therapeutic applications: mobilization of hematopoietic stem cells (HSCs) for autologous transplantations and treatment of solid tumors [84]. For the treatment of solid tumors, balixafortide acts in three different ways: firstly, it increases the number of immune cells such as B and T cells and natural killer cells (on which the CXCR4 receptor is expressed) by mobilizing these cells from the bone marrow to the peripheral circulatory system, increasing the effectiveness of immunotherapy [130]; secondly, it antagonizes the CXCR4 receptor on immunosuppressive cells, thereby enhancing the natural immunosuppression of a tumor environment and modulating the tumor microenvironment [84]. Furthermore, it causes the infiltration of effector cells in the tumor microenvironment. All these results make motixafortide a promising immune-modulatory agent with potent antitumor effects [132]. This peptide completed a phase II clinical trial whose first part consisted of studying the mechanism of motixafortide administered in combination with pembrolizumab in patients with metastatic pancreatic adenocarcinoma, and the second part consisted of studying the safety, tolerability, and efficacy of motixafortide when administered in conjunction with pembrolizumab and chemotherapy. The results were promising, and the next step will be to conduct another trial to confirm the data before moving on to phase III clinical trials [130]. In 2020, another phase-II clinical trial was initiated to evaluate the administration of motixafortide with cemiplimab and standard-of-care chemotherapy in patients with metastatic pancreatic ductal adenocarcinoma [84].
Regarding the mobilization of HSCs, motixafortide has completed phase-III clinical trials and is in the pre-submission phase. It is expected to obtain FDA approval and begin commercialization of motixafortide as a stem cell mobilization agent for bone marrow transplantation in multiple myeloma patients as early as 2023 [84].
2.3. BT1718
BT1718 is a bicycle drug conjugate with antitumor activity developed by Bicycle Therapeutics. This conjugate is composed of a 13–amino acids bicyclic peptide covalently linked to a toxin (DM1), a potent anti-tubulin agent [86][87]. The peptide part of this conjugate binds with high affinity and specificity to membrane type I matrix metalloproteinase (MT1-MMP) [87]. MT1-MMP is a metalloprotease that is overexpressed in several solid tumors and is directly related to tumor invasion and metastasis [85]. This metalloprotease is used as a target to facilitate the delivery of DM1 [133]. BT1718 was obtained from libraries of compounds generated by phage display. These peptide sequences were then cyclized with a TBMB scaffold, and those with the highest affinity for MT1 were identified through a high-throughput selection process. These peptides were further optimized with the introduction of non-natural amino acids to improve the plasma stability of the peptide [85].
BT1718 is currently in phase I/IIa clinical trial in which the maximum safe dose, the potential side effects, and pharmacokinetic properties in patients with advanced solid tumors are being studied (ClinicalTrials.gov Identifier: NCT03486730) [86].
2.4. BT8009
BT8009 is a bicyclic drug conjugate developed by Bicycle Therapeutics composed of a 14–amino acids bicyclic peptide targeting Nectin-4 linked to monomethyl auristatin E (MMAE), a cytotoxin by a valine–citrulline linker [89]. Nectin-4 is a molecule that is overexpressed in certain types of tumors but limited in healthy tissues [88]. The peptide binds to Nectin-4 on the cell surface, and the cytotoxin is released via a peptidase. The binding between the peptide and Nectin-4 revealed a high affinity [88]. The peptide was identified using phage display technology and was further synthesized to increase affinity and improve its hydrophilicity and stability [88]. A phase I/II clinical trial is currently underway, in which its administration in combination with nivolumab is being studied to assess safety, efficacy, and possible adverse effects (NCT04561362) [89].
2.5. BT5528
BT5528 is another bicyclic peptide conjugated to MMAE via a cleavable linker with antitumor activity developed by Bicycle Therapeutics. In this way, the 14-amino acids bicyclic peptide binds to EphA2 and delivers MMAE mainly in cancer cells [90]. Similar to peptides of the two previous conjugates developed by Bicycle Therapeutics, this bicyclic peptide was obtained by phage display screen against EphA2. Cyclization of the phage display generated a peptide sequence that was further cyclized using 1,3,5-triacryoyl-1,3,5-triazinane, a more hydrophilic scaffold than TBMB followed by lead optimization to improve stability and increase hydrophilicity using non-natural and polar charged amino acids [134]. Its synthesis was performed on Rink amide resin using Fmoc solid-phase synthesis guided by X-ray crystallography studies [134]. This peptide is now in phase I/II clinical trial to evaluate the safety and tolerability of BT5528 alone and in conjunction with nivolumab in patients with advanced solid tumors associated with EphA2 expression (NCT04180371) [135].
2.6. VT1021
VT1021 is a cyclic pentapeptide developed by Vigeo Pharmaceuticals with antitumor activity. This peptide is derived from prosaposin (a glycoprotein) that stimulates the production of thrombospondin-1 (Tsp-1) in myeloid-derived suppressor cells (MDSCs). Tsp-1 binds to CD36 of M2 macrophages, transforming them into M1 macrophages with anticancer action [136]. Tsp-1 also binds to tumor cells that express the CD36 receptor, inducing their death by apoptosis. This peptide activates cytotoxic T lymphocytes and inhibits angiogenesis [137][138]. In this way, the tumor microenvironment is reprogrammed, becoming an inhibitory environment for the tumor. Thus, this mechanism of action may be a successful approach for the treatment of tumors that have not responded to conventional chemotherapy [139].
In preclinical trials, this peptide demonstrated strong antitumor activity in animal models of ovarian, pancreatic, and breast cancer and exhibited the ability to cause tumor regression and reprogram the tumor microenvironment [137]. VT1021 has completed the phase I/II clinical trial safety, pharmacology, and preliminary efficacy in patients with advanced solid tumors (ovarian, pancreatic, triple-negative breast cancer, glioblastoma, and tumors that overexpress CD36) that did not respond to conventional therapies (ClinicalTrials.gov Identifier: NCT03364400). VT1021 was demonstrated to be safe and well-tolerated and showed activity in reprogramming the tumor microenvironment in patients expressing the CD36 receptor [91][136]. Due to these positive results, Vigeo announced in January of 2022 that VT1021 will advance to a phase II/III clinical trial in patients with glioblastoma that is already underway (ClinicalTrials.gov Identifier: NCT03970447). Furthermore, it is planned to proceed with efficacy studies in additional solid tumor indications [140].
2.7. ALRN-6924
ALRN-6924 is a synthetic cell-penetrating stapled α-helical peptide with 18 amino acids that mimics the transactivation domain of α-helix of p53, thus binding with high affinity to murine double minute 4 protein (MDMX) and murine double minute 2 protein (MDM2) (inhibitory constant (Ki) values of 24.7 and 7.7 nM, respectively) [141], thus inhibiting MDMX/p53 and MDM2/p53 protein–protein interactions [142][143].
The ALRN-6924 peptide is an analog of the ATSP-7041 [144] peptide that was the lead compound in a series of compounds emerging from phage display peptide optimization. The ATSP-7041 analog was synthesized using the Fmoc SPPS strategy. The linear peptide was synthesized using HCTU/6-Cl-1-hydroxybenzotriazole (HOBt). Then, the olefin building block was bounded and introduced with HATU/HOAt. The Fmoc groups were removed with 20% (v/v) piperidine/DMF, and the N-terminal amine was acetylated. Finally, cyclization was performed with the linear peptide still bound to the resin. Lastly, the resin was removed, and the final product was purified by liquid chromatography-mass spectrometry (LC-MS) [145].
This peptide has been developed by Aileron Therapeutics as a chemoprotective agent for healthy cells to minimize the adverse effects caused by chemotherapy [146]. This is possible by taking advantage of the fact that the TP53 gene that encodes the p53 protein is mutated in 50% of cancers [142]. That is, ALRN-6924 binds to MDMX and MDM2 and leaves p53 free, thus temporarily activating cell-cycle arrest. This binding only occurs in cells in which p53 is not mutated, in healthy cells. Therefore, tumor cells continue to be active and remain susceptible to the action of antitumor agents [147]. In this way, this peptide makes it possible to improve the effectiveness of conventional chemotherapeutic agents, protecting healthy cells and thus improving the usual side effects [92]. In tumors where the p53 mutation is rare, ALRN-6924 has also been shown to be useful since releasing p53 from the MDMX and MDM2 proteins induces cell cycle arrest and subsequent apoptosis of these tumor cells [148].
Currently, ALRN-6924 is in phase Ib clinical trials in which its safety, tolerability, and chemoprotective effect are being studied in combination with paclitaxel in patients with advanced, metastatic, or unresectable solid tumors (ClinicalTrials.gov identifier: NCT03725436) [149].
2.8. CEND-1
CEND-1 is an eight-amino acid cyclic peptide developed by CEND Therapeutics. This peptide was identified by phage display from a library of cyclic peptides whose cyclization occurred between two Cys residues and was shown to recognize tumor blood vessels in experimental metastasis mouse models of human prostate cancer [150]. The strategy used aimed to increase the tissue penetration of a tumor-targeted antitumor drug and decrease the resistance to antitumor drugs created by the high density of tissue around the tumor [93]. This is possible thanks to the presence of an RGD motif that selectively binds to the β5 integrin that is present exclusively in the tumor blood vessels and not in the blood vessels of healthy tissues [151]. Once bound to integrin, CEND-1 undergoes the action of a proteolytic enzyme that gives rise to the CendR portion, which increases the binding affinity to neuropillin-1, activating a transcytotic pathway in endothelial cells that increases the increased vascular transcytosis and permeability. For these reasons, this peptide allows the possibility to be used as an antitumor drug to reach tumor cells more easily [93].
Currently, a phase II clinical trial is underway, which is intended to study the effect of CEND-1 when administered with chemotherapy in patients with untreated metastatic pancreatic ductal adenocarcinoma (ClinicalTrials.gov Identifier: NCT05042128) [152].
2.9. POL7080—Inhaled Murepavadin
Murepavadin (POL 7080) is a fully synthetic cyclic peptide with 14 amino acid residues developed by Spexis [153]. This peptide has a unique antimicrobial activity being the first member of a new class of antibiotics with novel mechanisms of action—Outer Membrane Protein Targeting Antibiotics (OMPTA)—developed by Polyphor in collaboration with the University of Zurich [154]. Murepavadin has a nonlytic mechanism of action, acting through binding to an outer membrane protein responsible for the biosynthesis of lipopolysaccharide in Gram-negative bacteria—lypopolysaccharide transport protein D (LptD). Through the link established with LptD, murepavadin inhibits the transport of lipopolysaccharide, causing changes in the bacterial outer membrane, which leads to cell death [153][155]. Murepavadin has a specific activity for Pseudomonas aeruginosa being explored for infections caused by this bacterium in patients with cystic fibrosis [153].
The development of this peptide was inspired by the host defense peptides, which have a broad spectrum of antimicrobial action and low resistance against Gram-negative bacteria—protegrin-I (a natural peptide with a β-sheet structure due to disulfide bridges) [156]. As these peptides had unfavorable properties to be used as drugs, the strategy was to synthesize a library of compounds inspired by protegrin-I using the PEM approach developed by Polyphor [157]. Compound libraries with improved plasma stability and drug-like properties were synthesized, where murepavadin emerged [158]. Synthesis of the analogs was carried out using SPPS to synthesize the linear precursor in a 2-chlorotrityl chloride resin using Fmoc chemistry. The cyclization was carried out in solution using N-[(dimethylamino)-1 H-1, 2,3-triazolo [4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HATU)/1-hydroxy-7-azabenzotriazole (HOAt) as coupling agent after cleavage of the resin. Purification was carried out by preparative HPLC to obtain the desired cyclic peptide [157][159].
In in vitro and in vivo preclinical trials, its activity against various Pseudomonas species and other Gram-negative bacteria, the potential development of resistance, pharmacodynamics, pharmacokinetics, and metabolism were studied [153][155]. The pre-clinical results were promising, which made this peptide advance to various phase-I and II clinical trials where its safety, efficacy, tolerability, pharmacokinetics, and pharmacodynamics, among other aspects, were tested [153][160]. However, the phase III trial was temporarily stopped due to safety concerns (ClinicalTrials.gov Identifier: NCT03409679). Considering these unexpected results, Spexis initiated, in 2021, phase-I clinical trials maintaining the same goal, the treatment of P. aeruginosa infections in patients with cystic fibrosis, but testing inhaled murepavadin [154]. P. aeruginosa is characterized by a biofilm-forming mode of growth, resistance to antibiotics, and a high capacity to mutate. This mode of administration may be a good strategy for the treatment of infections caused by this microorganism. One of the reasons is that it acts immediately in the lungs without having to undergo systemic exposure, which also allows adverse effects to be minimized [94].
2.10. Thanatin Derivatives
In 2019, Spexis, together with the University of Zurich, started a program to develop the thanatin derivatives [154]. Thanatin is an insect-derived 21 amino acid residue peptide that shows activity against Gram-positive and Gram-negative bacteria, as well as against some fungi [161]. This antibiotic belongs to the new OMPTA class, but unlike murepavadin, it binds to lypopolysaccharide transport protein A (LptA), inhibiting lipopolysaccharide transport and causing damage to the microorganism’s outer membrane [162].
Thanatin has been undergoing optimization to improve its low in vitro plasma stability and its low half-life time in vivo. The successive optimizations have resulted in several thanatin derivatives with improved drug-like properties. This process is being developed by Spexis with funding from CARB-X [154].
2.11. CD-101—Rezafungin
Rezafungin (CD-101) is a new semisynthetic echinocandin that was under development for the treatment of fungal infections of Candida and Aspergillus spp. [163]. Rezafungin is an anidulafungin analog, which is considered a second-generation echinocandin that presents improved pharmacokinetic and pharmacodynamic parameters. It also has an improved tissue penetration and a good safety profile [164][165]. The mechanism of action of rezafungin is similar to other echinocandins; it is an inhibitor of cell wall synthesis by inhibition of 1,3-β-D-glucan synthesis [164]. Rezafungin completed a phase-III clinical trial that proved to be safe and effective in the treatment of candidemia and/or invasive candidiasis (ClinicalTrials.gov Identifier: NCT03667690) and is currently participating in a phase-III clinical trial to assess the efficacy and safety in the prevention of invasive fungal diseases (ClinicalTrials.gov Identifier: NCT04368559). Recently, rezafungin for injection was approved by FDA to treat candidemia and invasive candidiasis in adults with limited or no alternative treatment options [27].
2.12. RA-101495—Zilucoplan
Zilucoplan (RA-101495) is a 15-amino acid macrocyclic synthetic peptide developed by Ra Pharmaceuticals (now UCB Pharma). It is an inhibitor of the C5 complement that binds allosterically with a subnanomolar affinity [97][166]. This peptide is under development for the treatment of paroxysmal nocturnal hemoglobinuria and generalized myasthenia gravis (gMG) [97]. Zilucoplan plays an important role in these two diseases since it binds with high affinity and specificity to complement component 5 (C5), preventing cleavage in C5a and C5b and the interaction between C5b and C6. In this way, the membrane attack complex (MAC) is not formed, and the cell membrane is not destroyed [166].
Zilucoplan was synthesized by SPPS Fmoc/tBu method. The synthesis was performed on a Liberty automated microwave peptide synthesizer. The coupling of the amino acids was performed using 2-(6-chloro-1-H-benzotriazole-lyl)-l,l,3,3,-tetramethylaminium hexafluorophosphate (HCTU) as a coupling agent and diisopropylethylamine (DIEA) as a base. The cyclization of zilucoplan was via a lactam bridge between two amino acids of the linear peptide [167].
Zilucoplan completed two phase-II clinical trials for therapeutic application in paroxysmal nocturnal hemoglobinuria that served to study the safety and efficacy of this drug in patients with this disease and in patients who had an inadequate response to eculizumab (ClinicalTrials.gov Identifier: NCT03030183). Another phase-II clinical trial started with this drug to evaluate the effect of its prolonged use in patients who completed the previous clinical trials; however, UCB Pharma decided to stop the investigation of zilucoplan for application in the paroxysmal nocturnal hemoglobinuria disease [168]. For the gMG application, zilucoplan completed a phase-II clinical trial (ClinicalTrials.gov identifier: NCT03315130) in 2020, which demonstrated effective complement inhibition and a favorable safety and tolerability profile [169]. This peptide has already successfully completed a phase-III clinical trial (ClinicalTrials.gov identifier: NCT04115293), in which its efficacy, safety, and tolerability in patients with gMG were confirmed [170]. Considering these positive results, UCB Pharma plans to move forward with regulatory submissions for this drug during the current year [171]. A phase-III clinical trial (ClinicalTrials.gov identifier: NCT04225871) is currently ongoing, which is an open-label extension study to assess the long-term safety, efficacy, and tolerability of zilucoplan in patients with gMG who have already completed a clinical trial with zilucoplan [168].
2.13. AP301—Solnatide
Solnatide (AP301) is a fully synthetic cyclic peptide with 17 amino acids developed by Apeptico [98][172]. This peptide is under development for the treatment of pulmonary permeability edema in patients with moderate-to-severe acute respiratory distress syndrome (ARDS). Regarding the route of administration, it is intended to be administered by inhalation of a liquid aerosol delivered directly to the lungs with minimal plasma absorption [173]. Its structure mimics the lectin-like domain of human tumor necrosis factor and has the ability to activate the amiloride-sensitive sodium channel (ENaC), which is the main driving force capable of absorbing water through the reabsorption of Na+ through the lung epithelium, thus promoting the alveolar liquid clearance [174]. In addition, solnatide is also able to reduce intracellular levels of reactive oxygen species, inhibit the activation of protein kinase C α, reduce the degree of phosphorylation of the myosin light chain, and inhibit the pro-inflammatory pathway by reducing the production of cytokines [173].
In a non-clinical trial phase, solnatide was demonstrated to promote alveolar liquid clearance through the activation of ENaC in dog, pig, and rat models, reduced the long injury in a pig model, and proved to be effective in primary graft dysfunction after transplantation in rats [175]. In a phase-I clinical trial, it exhibited a safe and well-tolerated profile in increasing single doses [176]. In a phase-IIa clinical trial, solnatide was demonstrated to be safe in patients with mild to severe ARDS and appeared to be more effective in sicker patients, this evidence will be confirmed in later clinical trials [177].
Although this peptide is still in phase II clinical trials, it has already been approved for use in patients with COVID-19 in Austria and Italy in June 2022. The reason was due to the urgent need for drugs to reduce mortality and the severity of the disease caused by the SARS-CoV-2 virus. Solnatide was tested in patients infected with this virus because its infection causes pulmonary permeability edema. Interestingly, it demonstrated an improvement in symptoms and an increased survival rate after 28 days [173].
2.14. POL6014—Lonodelestat
Lonodelestat (POL6014) is a synthetic 13-amino-acid cyclic peptide developed by Polyphor through PEM technology [99]. Development and commercialization rights for this peptide were obtained by Santhera Pharmaceuticals from Polyphor. POL6014 was synthesized by SPPS and is in development for cystic fibrosis, acting as a potent and selective inhibitor of neutrophil elastase. By inhibiting this enzyme, the number of neutrophils released is reduced, thus decreasing inflammation and lung tissue damage, which preserves lung function [99]. This peptide is administered by inhalation [178]. Lonodelestat ended phase Ib/IIa clinical trial in December 2020 (ClinicalTrials.gov Identifier: NCT03748199).
2.15. THR-149
THR-149 is a bicyclic peptide developed by Oxurion NV that prevents pathologies associated with diabetic macular edema (DME). This peptide is an inhibitor of plasma kallikrein (PKal) that leads to the production of mediators of inflammation, vasodilation, and increased vessel permeability leading to microvascular retinal damage, among other effects [101]. THR-149 emerged from a library of PKaI inhibitor compounds obtained by phage display technology combined with a chemical cyclization [100]. THR-149 was the most potent and stable [101]. The synthesis process of this peptide starts with the synthesis of the linear peptide by Fmoc SPPS using Symphony automated peptide synthesizer and Rink amide resin. Then, the peptide was cleaved from the resin, precipitated, and lyophilized. The final step involved cyclization with TBMB [100].
Currently, this peptide is in a phase IIb clinical trial where its safety and efficacy are being evaluated (ClinicalTrials.gov Identifier: NCT04527107).
2.16. PTG-300—Rusfertide
Rusfertide (PTG-300) is a synthetic 18-amino acids hepcidin mimetic peptide developed by Protagonist Therapeutics [179]. This peptide acts on ferroportin, an iron transporter, causing its internalization and degradation [179]. This effect causes a decrease in the level of iron in the serum and transferrin saturation because it prevents iron from leaving the cells responsible for its storage [102][179]. This peptide is under development for the treatment of symptoms of polycythemia vera, a rare hematologic disease in which erythropoiesis occurs at very high levels, which increases the risk of thrombosis and causes iron deficiency [102][180]. Due to its proven activity in iron regulation, PTG-300 is also being studied for the treatment of hereditary hemochromatosis, a disease characterized by excessive absorption of iron by cells that leads to the degradation of organs, such as the liver, heart, and pancreas [103].
Currently, a phase-III clinical trial is ongoing to evaluate the safety and efficacy of PTG-300 in the improvement of polycythemia vera symptoms (NCT05210790) [181].
2.17. PN-943
PN-943 is a cyclic peptide α4β7 integrin antagonist developed by Protagonist Therapeutics for the treatment of ulcerative colitis [104][105]. α4β7 is present on the surface of memory T and B lymphocytes, associated with the recruitment of leukocytes to the intestinal mucosa [105]. This peptide prevents the binding of α4β7 integrin to the mucosal addressin cell adhesion molecule that is normally expressed in high values in inflamed GI tissues, which leads to the impediment of leukocyte recruitment and, consequently, to the reduction in GI inflammation [105].
PN-943 was found to be stable in the GI tract after oral administration, which was attractive because its site of action is in the GI tract [105]. This drug is in phase-II clinical trials to evaluate the safety, tolerability, and clinical efficacy of two different doses in patients with moderate to severe active ulcerative colitis (ClinicalTrials.gov Identifier: NCT04504383).
2.18. PL8177
PL8177 is a synthetic cyclic seven-amino-acid peptide that acts as a potent and selective agonist of the melanocortin 1 receptor developed by Palatin Technologies [106]. It is an analog of α-melanocyte-stimulating hormone, which is a peptide that regulates central and peripheral inflammation. Thus, this peptide is under development for the treatment of ulcerative colitis by oral administration [106][182]. This peptide contains a D-phenylalanine (Phe), a strategy used to improve the pharmacokinetic properties of peptides [106].
A phase-II clinical trial is underway to study its safety, tolerability, efficacy, pharmacokinetics, and biomarkers of oral administration in patients with ulcerative colitis (ClinicalTrials.gov Identifier: NCT05466890).
2.19. PA-001
PA-001 is a constrained cyclic peptide initially discovered by PeptiDream, but which has been optimized and selected by PeptiAID [107]. This peptide showed very strong activity against the original SARS-CoV-2 and its α, β, γ, and δ mutations. Furthermore, this peptide demonstrated synergistic effects when administered in combination with other drugs approved for emergency use for COVID-19 [107]. In pre-clinical trials, it has shown great safety [107]. In February 2022, this peptide began initial clinical trials in Japan, where it demonstrated favorable safety and pharmacokinetic results. In addition, it was also shown to be effective for the omicron variant of the SARS-CoV-2 virus (Japan Registry of Clinical Trials Trial ID: jRCTs031210601) [183]. Currently, PeptiAID intends to start a phase-I clinical trial in the United States of America and a phase-I/IIa clinical trial in Japan [183]. PeptiDream uses the Peptide Discovery Platform System, where libraries of compounds are rapidly generated by translating a DNA library into peptides and then tests the affinity of those peptides against the intended target [184].
2.20. AZP-3813
AZP-3813 is a macrocyclic peptide composed of 16 amino acids that acts as a growth hormone receptor antagonist. It is under development for the treatment of acromegaly, which is caused by excess secretion of growth hormone, which stimulates excess production of insulin-like growth factor 1 (IGF1). This peptide is derived from peptide sequences discovered using a cell-free in vitro transcription–translation system that was then optimized by rational design [108][185]. In preclinical trials, this peptide has been shown to be effective in decreasing IGF1 levels, which shows its potential to develop a drug against acromegaly [185].
2.21. PL9643
PL9643 is a synthetic cyclic peptide which is a melanocortin receptor agonist developed for the treatment of dry eye disease [109]. This peptide is in phase III clinical trials to evaluate the safety and efficacy of its ophthalmic administration to patients with dry eye disease (ClinicalTrials.gov Identifier: NCT05201170).
In addition to the peptides as new chemical entities, there are also cases of repositioning of cyclic peptides, that is, approved cyclic peptides that are in clinical trials for the treatment of other diseases. The structures of these peptides are shown in Figure 4.
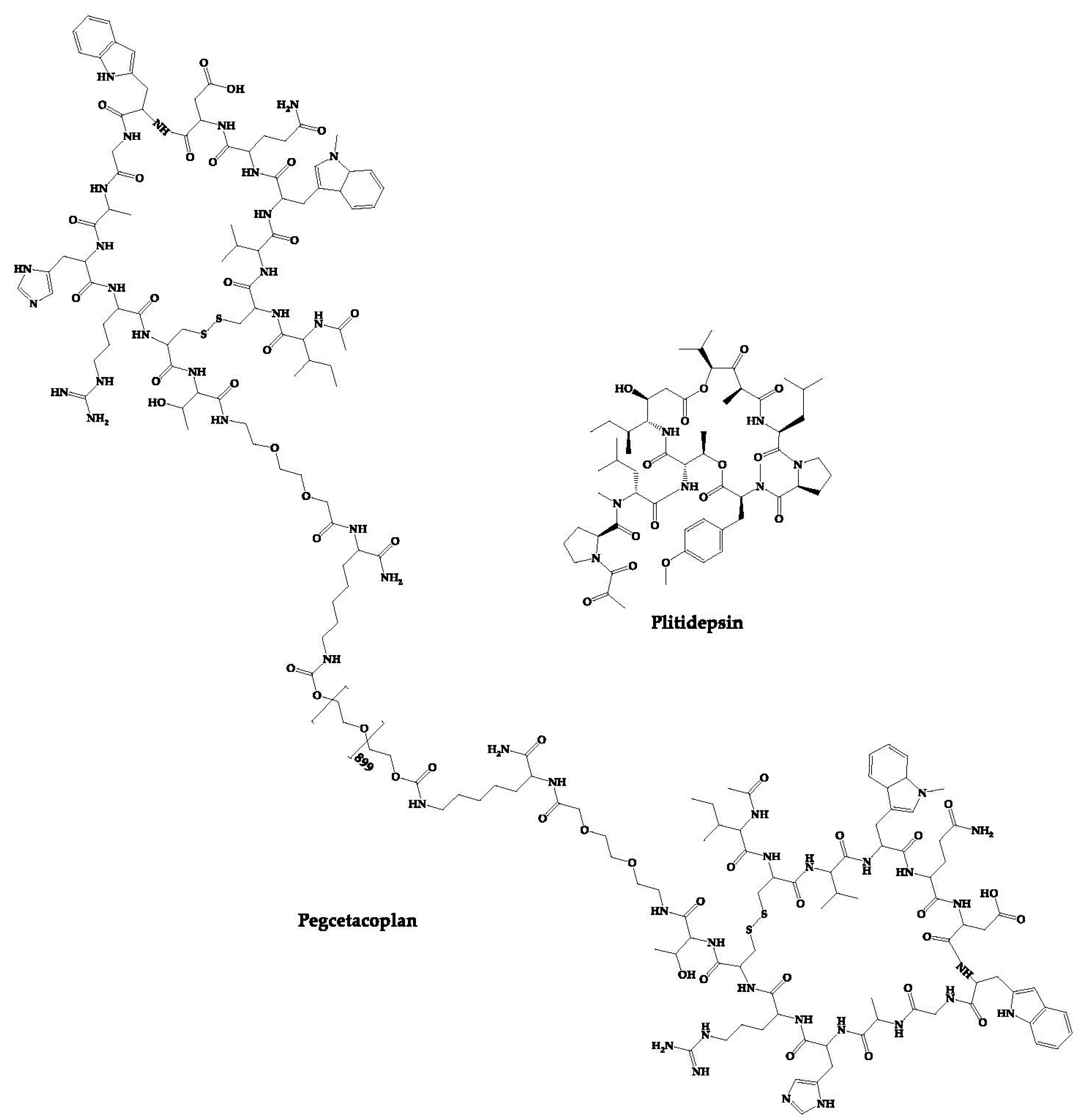
Figure 4. Structures of approved cyclic peptides currently in clinical trials for other diseases.
2.22. PM90001—Plitidepsin
Plitidepsin (PM90001) is a marine-derived depsipeptide that was approved in Australia for the treatment of multiple myeloma [110]. The Plitidepsin discovery process started with its isolation from a Mediterranean marine tunicate (Aplidium albicans) and later was fully synthesized [110]. PharmaMar has developed this peptide, which interacts with eukaryotic Elongation Factor 1A2 (eEF1A2), thus showing antitumor action by cell cycle arrest, apoptosis, and growth inhibition [186]. In addition to its approved activity, it has also demonstrated activity against the SARS-CoV-2 virus. This peptide inhibits the host eEF1A2 used by the SARS-CoV-2 virus to replicate. Thus, plitidepsin has demonstrated in vitro an inhibitory effect on the replication of this virus [46].
Currently, plitidepsin is in phase-II clinical trials to evaluate its efficacy in immunocompromised patients with symptomatic COVID-19 (ClinicalTrials.gov Identifier: NCT05705167).
2.23. APL-2—Pegcetacoplan
Pegcetacoplan (APL-2) is composed of two cyclic peptides linked together by a linear chain of polyethylene glycol molecules that increases its half-life [187]. It is a C3 inhibitor developed by Appelis and was approved for the treatment of paroxysmal nocturnal hemoglobinuria in May 2021 [111]. Clinical trials are currently underway with pegcetacoplan in phases II and III of development for diseases such as amyotrophic lateral sclerosis (ALS), immune-complex membranoproliferative glomerulonephritis and C3 glomerulopathy (IC-MPGN and C3G), cold agglutinin disease (CAD) and hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA) [188].
There are cases of cyclic peptides whose development has been interrupted (Figure 5) for various reasons. Some examples are mentioned in this sub-chapter.
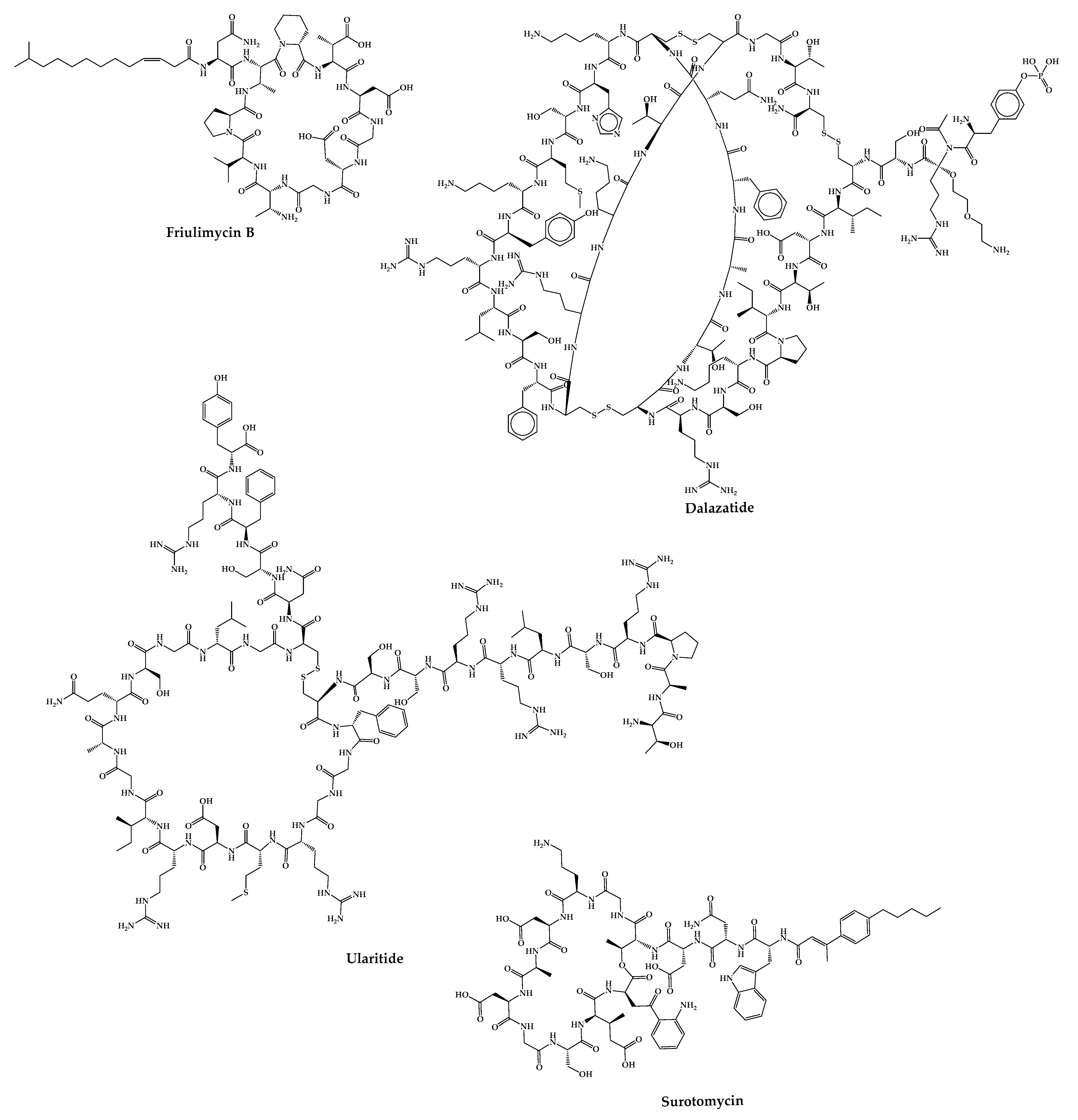
Figure 5. Structures of cyclic peptides in clinical trials whose development has been interrupted.
2.24. Friulimicin B
Friulimicin B is a natural cyclic lipopeptide with 11 amino acids produced by the actinomycete Actinoplanes friuliensis with antibacterial activity against a broad range of Gram-positive bacteria, including antibiotic-resistant pathogens [112]. Its mechanism of action is based on blocking cell wall biosynthesis. Structurally, this peptide consists of a macrocyclic decapeptide with a lipidic tail [113]. This peptide did not successfully complete the phase I clinical trial due to its poor pharmacokinetic properties [189].
2.25. ShK-186—Dalazatide
Dalazatide (Shk-186) is a synthetic 37-amino-acid cyclic peptide that inhibits the voltage-gated Kv1.3 potassium channel and is developed to treat plaque psoriasis [115]. This peptide is a synthetic analog of ShK (a natural peptide toxin isolated from the venom of the sea anemone Stichodactyla helianthus) [190][191]. Dalazatide was synthesized using the Fmoc-SPPS strategy on an amide resin. Couplings were performed with 6-Cl-N-HOBt in the presence of DIC. The removal of the Fmoc group was carried out with piperidine in DMF containing HOBt. Cleavage of the peptide from the resin was achieved with TFA. The disulfide bridge formation was achieved by air oxidation or through the addition of a glutathione exchange system [192]. This molecule successfully completed the phase Ib clinical trial in 2015 (ClinicalTrials.gov Identifier: NCT02435342), but it was not entered in any further clinical trials [115]. However, Kv 1.3 therapeutics claims to have the peptide ready to start a phase II clinical trial for the treatment of inclusion body myositis [193].
2.26. Ularitide
Ularitide, a peptide with 32 amino acids, is the synthetic form of urodilatin, a human natriuretic peptide synthesized in the distal kidney tubules that has been isolated from human urine [116][194]. Cardiorentis is working on the development of this peptide for the treatment of acute decompensated heart failure, a disease characterized by the sudden onset of symptoms of heart failure being responsible for many deaths and morbidity [117][195].
In phase-I clinical trials, ularitide had shown positive results in vasodilation and renal protection [196]. In phase-II clinical trials, its safety and efficacy were studied, which demonstrated that ularitide decreases cardiac filling pressures, improves dysponea, and protects short-term renal function. Furthermore, ularitide demonstrated a good safety profile [197]. In the phase-III clinical trial, ularitide decreased cardiac-wall stress; however, it did not reduce myocardial mortality and did not influence disease progression [198].
2.27. CB-183,315—Surotomycin
Surotomycin (CB-183,315) is a 13-amino acid cyclic lipopeptide analog of daptomycin with a different tail. Similar to daptomycin, surotomycin shows antibacterial activity against Clostridium difficile through disruption of the cell membrane [199]. This peptide completed phase-III clinical trials; however, it did not advance in the pipeline because the results showed that surotomycin did not demonstrate superiority over vancomycin in terms of clinical response over time [114].
This entry is adapted from the peer-reviewed paper 10.3390/ph16070996
References
- de la Torre, B.G.; Albericio, F. Peptide Therapeutics 2.0. Molecules 2020, 25, 2293.
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, R.O. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm. 2020, 587, 119491.
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531.
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445.
- Zhang, H.; Chen, S. Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chem. Biol. 2022, 3, 18–31.
- Rangel, M.; Santana, C.; Pinheiro, A.; Anjos, L.; Barth, T.; Pires Júnior, O.; Fontes, W.; S Castro, M. Marine Depsipeptides as Promising Pharmacotherapeutic Agents. Curr. Protein Pept. Sci. 2016, 17.
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.u. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30.
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143.
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33.
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957.
- Benția, D.; Saceleanu, M.V.; Marinescu, A.A.; Ciurea, A.V. Centenary of Insulin Discovery (1921–2021): Nicolae Paulescu’s Original Contributions. Acta Endocrinol. 2021, 17, 406–411.
- Ueno, H.; Zhang, W.; Nakazato, M. Regulation of feeding and therapeutic application of bioactive peptides. Pharmacol. Ther. 2022, 239, 108187.
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Biorg. Med. Chem. 2018, 26, 2700–2707.
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325.
- Ruoslahti, E.; Pierschbacher, M.D. New Perspectives in Cell Adhesion: RGD and Integrins. Science 1987, 238, 491–497.
- Storgard, C.M.; Stupack, D.G.; Jonczyk, A.; Goodman, S.L.; Fox, R.I.; Cheresh, D.A. Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J. Clin. Investig. 1999, 103, 47–54.
- Mas-Moruno, C.; Rechenmacher, F.; Kessler, H. Cilengitide: The first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med. Chem. 2010, 10, 753–768.
- Beer, A.J.; Haubner, R.; Goebel, M.; Luderschmidt, S.; Spilker, M.E.; Wester, H.-J.; Weber, W.A.; Schwaiger, M. Biodistribution and Pharmacokinetics of the αvβ3-Selective Tracer 18F-Galacto-RGD in Cancer Patients. J. Nucl. Med. 2005, 46, 1333–1341.
- Choi, J.S.; Joo, S.H. Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2020, 28, 18–24.
- PepTherDia. Available online: http://peptherdia.herokuapp.com/ (accessed on 2 May 2023).
- D’Aloisio, V.; Dognini, P.; Hutcheon, G.A.; Coxon, C.R. PepTherDia: Database and structural composition analysis of approved peptide therapeutics and diagnostics. Drug Discov. Today 2021, 26, 1409–1419.
- Gall, Y.M.; Konashev, M.B. The discovery of Gramicidin S: The Intellectual Transformation of G.F. Gause from Biologist to Researcher of Antibiotics and on its Meaning for the Fate of Russian Genetics. Hist. Philos. Life Sci. 2001, 23, 137–150.
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and its use in the Treatment of Infected Wounds. Nature 1944, 154, 703.
- Klinker, K.P.; Borgert, S.J. Beyond Vancomycin: The Tail of the Lipoglycopeptides. Clin. Ther. 2015, 37, 2619–2636.
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61, S604–S611.
- Jain, S.; Zain, J. Romidepsin in the treatment of cutaneous T-cell lymphoma. J. Blood Med. 2011, 2, 37–47.
- Syed, Y.Y. Rezafungin: First Approval. Drugs 2023, 83, 833–840.
- Du, Z.; Fan, B.; Dai, Q.; Wang, L.; Guo, J.; Ye, Z.; Cui, N.; Chen, J.; Tan, K.; Li, R.; et al. Supramolecular peptide nanostructures: Self-assembly and biomedical applications. Giant 2022, 9, 100082.
- Panigrahi, B.; Singh, R.K.; Suryakant, U.; Mishra, S.; Potnis, A.A.; Jena, A.B.; Kerry, R.G.; Rajaram, H.; Ghosh, S.K.; Mandal, D. Cyclic peptides nanospheres: A ‘2-in-1′ self-assembled delivery system for targeting nucleus and cytoplasm. Eur. J. Pharm. Sci. 2022, 171, 106125.
- Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules 2021, 26, 3380.
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865.
- Armstrong, D.W.; Tang, Y.; Chen, S.; Zhou, Y.; Bagwill, C.; Chen, J.-R. Macrocyclic Antibiotics as a New Class of Chiral Selectors for Liquid Chromatography. Anal. Chem. 1994, 66, 1473–1484.
- Fernandes, C.; Tiritan, M.E.; Cass, Q.; Kairys, V.; Fernandes, M.X.; Pinto, M. Enantioseparation and chiral recognition mechanism of new chiral derivatives of xanthones on macrocyclic antibiotic stationary phases. J. Chromatogr. A 2012, 1241, 60–68.
- Phyo, Y.Z.; Cravo, S.; Palmeira, A.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M.; Fernandes, C. Enantiomeric Resolution and Docking Studies of Chiral Xanthonic Derivatives on Chirobiotic Columns. Molecules 2018, 23, 142.
- Mustafa, K.; Kanwal, J.; Musaddiq, S.; Khakwani, S. Bioactive Peptides and Their Natural Sources. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 75–97.
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208.
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392.
- Robinson, S.D.; Undheim, E.A.B.; Ueberheide, B.; King, G.F. Venom peptides as therapeutics: Advances, challenges and the future of venom-peptide discovery. Expert Rev. Proteom. 2017, 14, 931–939.
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802.
- Daly, N.L.; Wilson, D.T. Plant derived cyclic peptides. Biochem. Soc. Trans. 2021, 49, 1279–1285.
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides. Molecules 2017, 22, 2069.
- Kisil, O.V.; Efimenko, T.A.; Efremenkova, O.V. Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects. Antibiotics 2021, 10, 1254.
- Debono, M.; Barnhart, M.; Carrell, C.B.; Hoffmann, J.A.; Occolowitz, J.L.; Abbott, B.J.; Fukuda, D.S.; Hamill, R.L.; Biemann, K.; Herlihy, W.C. A21978C, a complex of new acidic peptide antibiotics: Isolation, chemistry, and mass spectral structure elucidation. J. Antibiot. 1987, 40, 761–777.
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459.
- Gu, H.; Han, S.M.; Park, K.-K. Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195.
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931.
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The Pharmacological Potential of Non-ribosomal Peptides from Marine Sponge and Tunicates. Front. Pharmacol. 2016, 7, 333.
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the Marine Sponge Callyspongia aerizusa: Cyclic Peptides with Antitubercular Activity. J. Nat. Prod. 2015, 78, 1910–1925.
- Xu, W.-J.; Liao, X.-J.; Xu, S.-H.; Diao, J.-Z.; Du, B.; Zhou, X.-L.; Pan, S.-S. Isolation, Structure Determination, and Synthesis of Galaxamide, A Rare Cytotoxic Cyclic Pentapeptide from a Marine Algae Galaxaura filamentosa. Org. Lett. 2008, 10, 4569–4572.
- Wibowo, J.T.; Bayu, A.; Aryati, W.D.; Fernandes, C.; Yanuar, A.; Kijjoa, A.; Putra, M.Y. Secondary Metabolites from Marine-Derived Bacteria with Antibiotic and Antibiofilm Activities against Drug-Resistant Pathogens. Mar. Drugs 2023, 21, 50.
- Pesic, A.; Baumann, H.I.; Kleinschmidt, K.; Ensle, P.; Wiese, J.; Süssmuth, R.D.; Imhoff, J.F. Champacyclin, a New Cyclic Octapeptide from Streptomyces Strain C42 Isolated from the Baltic Sea. Mar. Drugs 2013, 11, 4834–4857.
- Linington, R.G.; González, J.; Ureña, L.-D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H. Venturamides A and B: Antimalarial Constituents of the Panamanian Marine Cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007, 70, 397–401.
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a Potent Cytotoxin and Chymotrypsin Inhibitor from the Marine Cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27.
- de Sá, J.D.M.; Kumla, D.; Dethoup, T.; Kijjoa, A. Bioactive Compounds from Terrestrial and Marine-Derived Fungi of the Genus Neosartorya &dagger. Molecules 2022, 27, 2351.
- Du, F.-Y.; Zhang, P.; Li, X.-M.; Li, C.-S.; Cui, C.-M.; Wang, B.-G. Cyclohexadepsipeptides of the Isaridin Class from the Marine-Derived Fungus Beauveria felina EN-135. J. Nat. Prod. 2014, 77, 1164–1169.
- Wyche, T.P.; Hou, Y.; Vazquez-Rivera, E.; Braun, D.; Bugni, T.S. Peptidolipins B–F, Antibacterial Lipopeptides from an Ascidian-Derived Nocardia sp. J. Nat. Prod. 2012, 75, 735–740.
- Amelia, T.S.M.; Suaberon, F.A.C.; Vad, J.; Fahmi, A.D.M.; Saludes, J.P.; Bhubalan, K. Recent Advances of Marine Sponge-Associated Microorganisms as a Source of Commercially Viable Natural Products. Mar. Biotechnol. 2022, 24, 492–512.
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinto, M.M.M.; Dethoup, T.; Silva, A.M.S.; Kijjoa, A. A New Cyclic Hexapeptide and a New Isocoumarin Derivative from the Marine Sponge-Associated Fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450.
- May Zin, W.W.; Buttachon, S.; Dethoup, T.; Fernandes, C.; Cravo, S.; Pinto, M.M.M.; Gales, L.; Pereira, J.A.; Silva, A.M.S.; Sekeroglu, N.; et al. New Cyclotetrapeptides and a New Diketopiperzine Derivative from the Marine Sponge-Associated Fungus Neosartorya glabra KUFA 0702. Mar. Drugs 2016, 14, 136.
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Peptides: Determination of Absolute Configuration Using Liquid Chromatography Methods and Evaluation of Bioactivities. Molecules 2018, 23, 306.
- Fernandes, C.; Ribeiro, R.; Pinto, M.; Kijjoa, A. Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review. Molecules 2023, 28, 615.
- Kang, H.K.; Choi, M.-C.; Seo, C.H.; Park, Y. Therapeutic Properties and Biological Benefits of Marine-Derived Anticancer Peptides. Int. J. Mol. Sci. 2018, 19, 919.
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine Cyclic Peptides: Antimicrobial Activity and Synthetic Strategies. Mar. Drugs 2022, 20, 397.
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468.
- McIntosh, M.; Cruz, L.J.; Hunkapiller, M.W.; Gray, W.R.; Olivera, B.M. Isolation and structure of a peptide toxin from the marine snail Conus magus. Arch. Biochem. Biophys. 1982, 218, 329–334.
- Kohli, R.M.; Walsh, C.T.; Burkart, M.D. Biomimetic synthesis and optimization of cyclic peptide antibiotics. Nature 2002, 418, 658–661.
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221.
- Jiang, S.; Li, Z.; Ding, K.; Roller, P.P. Recent Progress of Synthetic Studies to Peptide and Peptidomimetic Cyclization. Curr. Org. Chem. 2008, 12, 1502–1542.
- Hamada, Y.; Shioiri, T. Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides. Chem. Rev. 2005, 105, 4441–4482.
- Anand, M.; Alagar, M.; Ranjitha, J.; Selvaraj, V. Total synthesis and anticancer activity of a cyclic heptapeptide from marine sponge using water soluble peptide coupling agent EDC. Arab. J. Chem. 2019, 12, 2782–2787.
- Ghosh, A.K.; Moon, D.K. Enantioselective Total Synthesis of (+)-Jasplakinolide. Org. Lett. 2007, 9, 2425–2427.
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810.
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203.
- Wallace, R.J. Acetylation of peptides inhibits their degradation by rumen micro-organisms. Br. J. Nutr. 1992, 68, 365–372.
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500.
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380.
- Trier, S.; Linderoth, L.; Bjerregaard, S.; Strauss, H.M.; Rahbek, U.L.; Andresen, T.L. Acylation of salmon calcitonin modulates in vitro intestinal peptide flux through membrane permeability enhancement. Eur. J. Pharm. Biopharm. 2015, 96, 329–337.
- Li, W.; O’Brien-Simpson, N.M.; Hossain, M.A.; Wade, J.D. The 9-Fluorenylmethoxycarbonyl (Fmoc) Group in Chemical Peptide Synthesis – Its Past, Present, and Future. Aust. J. Chem. 2020, 73, 271–276.
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 2006, 33, 239–254.
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; et al. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 24, 975–1020.
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018.
- Lamers, C. Overcoming the shortcomings of peptide-based therapeutics. Future Drug Discov. 2022, 4, FDD75.
- Zimmermann, J.; Klimkait, T.; Briand, F.; Obrecht, D. Dual anti-viral and immunomodulatory activity of the CXCR4 inhibitor Balixafortide (POL6326) in preclinical in vitro and in vivo SARS-CoV2 infection models. Swiss Med. Wkly. 2021, 151, 14S.
- Motixafortide (BL-8040). Available online: https://www.biolinerx.com/pipeline/bl-8040/overview (accessed on 12 February 2023).
- Harrison, H.; Bennett, G.; Blakeley, D.M.; Brown, A.; Campbell, S.; Chen, L.; Lutz, R.J.; Pavan, S.; Rietschoten, K.v.; Teufel, D.P.; et al. Abstract 5144: BT1718, a novel bicyclic peptide-maytansinoid conjugate targeting MT1-MMP for the treatment of solid tumors: Design of bicyclic peptide and linker selection. Cancer Res. 2017, 77, 5144.
- Cook, N.; Banerji, U.; Evans, J.; Biondo, A.; Germetaki, T.; Randhawa, M.; Godfrey, L.; Leslie, S.; Jeffrey, P.; Rigby, M.; et al. 464P—Pharmacokinetic (PK) assessment of BT1718: A phase I/II a study of BT1718, a first in class bicycle toxin conjugate (BTC), in patients (pts) with advanced solid tumours. Ann. Oncol. 2019, 30, v174.
- Bennett, G.; Lutz, R.; Park, P.; Harrison, H.; Lee, K. Abstract 1167: Development of BT1718, a novel Bicycle Drug Conjugate for the treatment of lung cancer. Cancer Res. 2017, 77, 1167.
- Rigby, M.; Bennett, G.; Chen, L.; Mudd, G.E.; Harrison, H.; Beswick, P.J.; Van Rietschoten, K.; Watcham, S.M.; Scott, H.S.; Brown, A.N.; et al. BT8009; A Nectin-4 Targeting Bicycle Toxin Conjugate for Treatment of Solid Tumors. Mol. Cancer Ther. 2022, 21, 1747–1756.
- Mudd, G.E.; Scott, H.; Chen, L.; van Rietschoten, K.; Ivanova-Berndt, G.; Dzionek, K.; Brown, A.; Watcham, S.; White, L.; Park, P.U.; et al. Discovery of BT8009: A Nectin-4 Targeting Bicycle Toxin Conjugate for the Treatment of Cancer. J. Med. Chem. 2022, 65, 14337–14347.
- Bennett, G.; Brown, A.; Mudd, G.; Huxley, P.; Van Rietschoten, K.; Pavan, S.; Chen, L.; Watcham, S.; Lahdenranta, J.; Keen, N. MMAE Delivery Using the Bicycle Toxin Conjugate BT5528. Mol. Cancer Ther. 2020, 19, 1385–1394.
- Mahalingam, D.; Harb, W.; Patnaik, A.; Ulahannan, S.; Mahdi, H.; Ahluwalia, M.; Patel, M.; Dowlati, A.; Bullock, A.; Wen, P.; et al. A first-in-human Phase 1/2 open label trial evaluating the safety, pharmacology, and preliminary efficacy of VT1021 in subjects with advanced solid tumors. J. Immunother. Cancer 2020, 8, A228.
- Pairawan, S.; Zhao, M.; Yuca, E.; Annis, A.; Evans, K.; Sutton, D.; Carvajal, L.; Ren, J.G.; Santiago, S.; Guerlavais, V.; et al. First in class dual MDM2/MDMX inhibitor ALRN-6924 enhances antitumor efficacy of chemotherapy in TP53 wild-type hormone receptor-positive breast cancer models. Breast Cancer Res. 2021, 23, 29.
- Hurtado de Mendoza, T.; Mose, E.S.; Botta, G.P.; Braun, G.B.; Kotamraju, V.R.; French, R.P.; Suzuki, K.; Miyamura, N.; Teesalu, T.; Ruoslahti, E.; et al. Tumor-penetrating therapy for β5 integrin-rich pancreas cancer. Nat. Commun. 2021, 12, 1541.
- Díez-Aguilar, M.; Hernández-García, M.; Morosini, M.-I.; Fluit, A.; Tunney, M.M.; Huertas, N.; del Campo, R.; Obrecht, D.; Bernardini, F.; Ekkelenkamp, M.; et al. Murepavadin antimicrobial activity against and resistance development in cystic fibrosis Pseudomonas aeruginosa isolates. J. Antimicrob. Chemother. 2020, 76, 984–992.
- Spexis Achieves First CARB-X Milestone for Its Thanatin Derivatives Program and Receives Funding of up to USD 1.9 Million to Initiate Lead Optimization. Available online: https://spexisbio.com/news-adhoc/news-detail/?newsid=2206705 (accessed on 8 March 2023).
- Krishnan, B.R.; James, K.D.; Polowy, K.; Bryant, B.J.; Vaidya, A.; Smith, S.; Laudeman, C.P. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J. Antibiot. 2017, 70, 130–135.
- Ricardo, A.; Arata, M.; DeMarco, S.; Dhamnaskar, K.; Hammer, R.; Fridkis-Hareli, M.; Rajagopal, V.; Seyb, K.; Tang, G.-Q.; Tobe, S.; et al. Preclinical Evaluation of RA101495, a Potent Cyclic Peptide Inhibitor of C5 for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Blood 2015, 126, 939.
- Zhou, Q.; Wang, D.; Liu, Y.; Yang, X.; Lucas, R.; Fischer, B. Solnatide Demonstrates Profound Therapeutic Activity in a Rat Model of Pulmonary Edema Induced by Acute Hypobaric Hypoxia and Exercise. Chest 2017, 151, 658–667.
- Barth, P.; Bruijnzeel, P.; Wach, A.; Sellier Kessler, O.; Hooftman, L.; Zimmermann, J.; Naue, N.; Huber, B.; Heimbeck, I.; Kappeler, D.; et al. Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J. Cyst. Fibros. 2020, 19, 299–304.
- Teufel, D.P.; Bennett, G.; Harrison, H.; van Rietschoten, K.; Pavan, S.; Stace, C.; Le Floch, F.; Van Bergen, T.; Vermassen, E.; Barbeaux, P.; et al. Stable and Long-Lasting, Novel Bicyclic Peptide Plasma Kallikrein Inhibitors for the Treatment of Diabetic Macular Edema. J. Med. Chem. 2018, 61, 2823–2836.
- Van Bergen, T.; Hu, T.T.; Little, K.; De Groef, L.; Moons, L.; Stitt, A.W.; Vermassen, E.; Feyen, J.H.M. Targeting Plasma Kallikrein with a Novel Bicyclic Peptide Inhibitor (THR-149) Reduces Retinal Thickening in a Diabetic Rat Model. Investig. Ophthalmol. Vis. Sci. 2021, 62, 18.
- Ginzburg, Y.; Kremyanskaya, M.; Kuykendall, A.T.; Yacoub, A.; Yang, J.; Gupta, S.K.; Valone, F.; Khanna, S.; Hoffman, R.; Verstovsek, S. Hepcidin Mimetic (PTG-300) Reverses Iron Deficiency While Controlling Hematocrit in Polycythemia Vera Patients. Blood 2020, 136, 40–41.
- Kowdley, K.V.; Modi, N.B.; Valone, F.; Priego, V.M.; Ferris, C.; Cole, F.; Gupta, S.K. Rusfertide (PTG-300), a Hepcidin Mimetic, Maintains Liver Iron Concentration in the Absence of Phlebotomies in Patients with Hereditary Hemochromatosis. Blood 2021, 138, 943.
- CHENG, L.; Venkataraman, S.; Zhao, L.; Lee, L.; Tang, T.; Liu, D.; Mattheakis, L. P004 PN-943, an oral α4β7 integrin antagonist, inhibits MAdCAM1-mediated proliferation and cytokine release from CD4+ T cells independent of trafficking. J. Crohn’s Colitis 2020, 14, S131.
- Modi, N.B.; Cheng, X.; Mattheakis, L.; Hwang, C.C.; Nawabi, R.; Liu, D.; Gupta, S. Single- and Multiple-Dose Pharmacokinetics and Pharmacodynamics of PN-943, a Gastrointestinal-Restricted Oral Peptide Antagonist of α4β7, in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2021, 10, 1263–1278.
- Dodd, J.; Jordan, R.; Makhlina, M.; Pesco Koplowitz, L.; Koplowitz, B.; Barnett, K.; Yang, W.H.; Spana, C. Pharmacokinetics of the Melanocortin Type 1 Receptor Agonist PL8177 After Subcutaneous Administration. Drugs RD 2021, 21, 431–443.
- PeptiDream Affiliated Company, PeptiAID Inc., Completes Preclinical Studies of PA-001 Candidate Compound for COVID-19 Therapeutics and Announces Future Plans. Available online: https://contents.xj-storage.jp/xcontents/45870/bfc69946/cf52/42a1/ab2f/c8908a52f8f8/20211111150641447s.pdf (accessed on 6 April 2023).
- Milano, S.; Kurasaki, H.; Tomiyama, T.; Reid, P.; Jan, V.; Culler, M. AZP-3813, a bicyclic 16-amino acid peptide antagonist of the human growth hormone receptor as a potential new treatment for acromegaly. Endocr. Abstr. 2022, 81, P148.
- Kenyon, K.; Ousler, G.W.; Watson, M.; Torkildsen, G.; Vollmer, P.; McLaurin, E.B.; Evans, D.; Winters, J.; Dodd, J.; Jordan, R.; et al. Efficacy and Safety of the Melanocortin Agonist PL9643 in a Phase 2 Study of Subjects with Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1333.
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Devel. Ther. 2017, 11, 253–264.
- Rehan, S.T.; Hashmi, M.R.; Asghar, M.S.; Tahir, M.J.; Yousaf, Z. Pegcetacoplan—A novel C3 inhibitor for paroxysmal nocturnal hemoglobinuria. Health Sci. Rep. 2022, 5, e512.
- Vértesy, L.; Ehlers, E.; Kogler, H.; Kurz, M.; Meiwes, J.; Seibert, G.; Vogel, M.; Hammann, P. Friulimicins: Novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. II. Isolation and structural characterization. J. Antibiot. 2000, 53, 816–827.
- Schneider, T.; Gries, K.; Josten, M.; Wiedemann, I.; Pelzer, S.; Labischinski, H.; Sahl, H.-G. The Lipopeptide Antibiotic Friulimicin B Inhibits Cell Wall Biosynthesis through Complex Formation with Bactoprenol Phosphate. Antimicrob. Agents Chemother. 2009, 53, 1610–1618.
- Daley, P.; Louie, T.; Lutz, J.E.; Khanna, S.; Stoutenburgh, U.; Jin, M.; Adedoyin, A.; Chesnel, L.; Guris, D.; Larson, K.B.; et al. Surotomycin versus vancomycin in adults with Clostridium difficile infection: Primary clinical outcomes from the second pivotal, randomized, double-blind, Phase 3 trial. J. Antimicrob. Chemother. 2017, 72, 3462–3470.
- Tarcha, E.J.; Olsen, C.M.; Probst, P.; Peckham, D.; Muñoz-Elías, E.J.; Kruger, J.G.; Iadonato, S.P. Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLoS ONE 2017, 12, e0180762.
- Anker, S.D.; Ponikowski, P.; Mitrovic, V.; Peacock, W.F.; Filippatos, G. Ularitide for the treatment of acute decompensated heart failure: From preclinical to clinical studies. Eur. Heart J. 2015, 36, 715–723.
- Emani, S.; Meyer, M.; Palm, D.; Holzmeister, J.; Haas, G.J. Ularitide: A natriuretic peptide candidate for the treatment of acutely decompensated heart failure. Future Cardiol. 2015, 11, 531–546.
- Kaufman, P.A.; Pernas, S.; Martin, M.; Gil-Martin, M.; Gomez Pardo, P.; Sara, L.T.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide (a CXCR4 antagonist) plus eribulin in HER2-negative metastatic breast cancer (MBC): A Phase I open-label trial. Eur. J. Cancer 2018, 92, S117–S118.
- Zimmermann, J.; Obrecht, D.; Remus, T. Abstract A003: Anti-angiogenic activity of the CXCR4 antagonist balixafortide. Mol. Cancer Ther. 2019, 18, A003.
- Schmitt, S.; Weinhold, N.; Dembowsky, K.; Neben, K.; Witzens-Harig, M.; Braun, M.; Klemmer, J.; Wuchter, P.; Ludin, C.; Ho, A.D.; et al. First Results of a Phase-II Study with the New CXCR4 Antagonist POL6326 to Mobilize Hematopoietic Stem Cells (HSC) In Multiple Myeloma (MM). Blood 2010, 116, 824.
- Tsomaia, N. Peptide therapeutics: Targeting the undruggable space. Eur. J. Med. Chem. 2015, 94, 459–470.
- Pernas, S.; Martin, M.; Kaufman, P.A.; Gil-Martin, M.; Gomez Pardo, P.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol. 2018, 19, 812–824.
- Kaufman, P.A.; Simon, S.P.; Martin, M.; Gil-Martin, M.; Pardo, P.G.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide (a CXCR4 antagonist) plus eribulin in HER2 negative metastatic breast cancer: Dose-response analysis of efficacy from phase I single-arm trial. J. Clin. Oncol. 2020, 38, e15209.
- Kaufman, P.A.; Martin, M.; Mayer, I.; Vahdat, L.T.; Simon, S.P.; Schmid, P.; McArthur, H.L.; Dent, R.; Rugo, H.S.; Barrios, C.; et al. 359TiP International phase III trial: Balixafortide (a CXCR4 antagonist) + eribulin versus eribulin alone in patients with HER2-negative, locally recurrent or metastatic breast cancer (FORTRESS). Ann. Oncol. 2020, 31, S394–S395.
- Polyphor Provides Final Update on the Phase III FORTRESS Study of Balixafortide in Patients with Advanced HER2 Negative Breast Cancer. Available online: https://spexisbio.com/news-adhoc/news-detail/?newsid=2139995 (accessed on 12 February 2023).
- Spexis’ CXCR4 Inhibitor Balixafortide Demonstrates Synergistic Efficacy in Combination with Docetaxel in a Metastatic Prostate Cancer Preclinical Model. Available online: https://spexisbio.com/news/corporate-news-details/?newsid=2295967 (accessed on 12 February 2023).
- Abraham, M.; Pereg, Y.; Bulvik, B.; Klein, S.; Mishalian, I.; Wald, H.; Eizenberg, O.; Beider, K.; Nagler, A.; Golan, R.; et al. Single Dose of the CXCR4 Antagonist BL-8040 Induces Rapid Mobilization for the Collection of Human CD34+ Cells in Healthy Volunteers. Clin. Cancer Res. 2017, 23, 6790–6801.
- Beider, K.; Begin, M.; Abraham, M.; Wald, H.; Weiss, I.D.; Wald, O.; Pikarsky, E.; Zeira, E.; Eizenberg, O.; Galun, E.; et al. CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp. Hematol. 2011, 39, 282–292.
- Burger, J.A.; Stewart, D.J.; Wald, O.; Peled, A. Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev. Anticancer Ther. 2011, 11, 621–630.
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885.
- Tamamura, H.; Xu, Y.; Hattori, T.; Zhang, X.; Arakaki, R.; Kanbara, K.; Omagari, A.; Otaka, A.; Ibuka, T.; Yamamoto, N.; et al. A Low-Molecular-Weight Inhibitor against the Chemokine Receptor CXCR4: A Strong Anti-HIV Peptide T140. Biochem. Biophys. Res. Commun. 1998, 253, 877–882.
- Gaur, P.; Verma, V.; Gupta, S.; Sorani, E.; Haras, A.V.; Oberkovitz, G.; Peled, A.; Khleif, S. CXCR4 antagonist (BL-8040) to enhance antitumor effects by increasing tumor infiltration of antigen-specific effector T-cells. J. Clin. Oncol. 2018, 36, 73.
- Gowland, C.; Berry, P.; Errington, J.; Jeffrey, P.; Bennett, G.; Godfrey, L.; Pittman, M.; Niewiarowski, A.; Symeonides, S.N.; Veal, G.J. Development of a LC–MS/MS method for the quantification of toxic payload DM1 cleaved from BT1718 in a Phase I study. Bioanalysis 2021, 13, 101–113.
- Mudd, G.E.; Brown, A.; Chen, L.; van Rietschoten, K.; Watcham, S.; Teufel, D.P.; Pavan, S.; Lani, R.; Huxley, P.; Bennett, G.S. Identification and Optimization of EphA2-Selective Bicycles for the Delivery of Cytotoxic Payloads. J. Med. Chem. 2020, 63, 4107–4116.
- Bendell, J.C.; Wang, J.S.-Z.; Bashir, B.; Richardson, D.L.; Bennett, G.; Campbell, C.; Hennessy, M.G.; Jeffrey, P.; Kirui, J.; Mahnke, L.; et al. BT5528-100 phase I/II study of the safety, pharmacokinetics, and preliminary clinical activity of BT5528 in patients with advanced malignancies associated with EphA2 expression. J. Clin. Oncol. 2020, 38, TPS3655.
- Mahalingam, D.; Mulcahy, M.; Juric, D.; Patel, M.; Pant, S.; Ulahannan, S.; Dowlati, A.; Bullock, A.; Vaickus, L.; Fyfe, S.; et al. Clinical update of VT1021, a first-in-class CD36 and CD47 targeting immunomodulating agent, in subjects with pancreatic cancer and other solid tumors stratified by novel biomarkers. J. Immunother. Cancer 2021, 9, A397.
- Harb, W.; Patnaik, A.; Mahalingam, D.; Liu, J.; Wen, P.Y.; Shapiro, G.I.; Bullock, A.J.; Juric, D.; Zheng, L.; Moore, K.; et al. 465P—A phase I open label dose escalation trial evaluating VT1021 in patients with advanced solid tumours. Ann. Oncol. 2019, 30, v175.
- Tanase, C.; Enciu, A.M.; Codrici, E.; Popescu, I.D.; Dudau, M.; Dobri, A.M.; Pop, S.; Mihai, S.; Gheorghișan-Gălățeanu, A.A.; Hinescu, M.E. Fatty Acids, CD36, Thrombospondin-1, and CD47 in Glioblastoma: Together and/or Separately? Int. J. Mol. Sci. 2022, 23, 604.
- OUR APPROACH. Available online: https://vigeotherapeutics.com/our-approach/overview/ (accessed on 12 February 2023).
- NEWS. Available online: https://vigeotherapeutics.com/news/vigeo-therapeutics-advances-vt1021-into-phase-2-3-registrational-study-for-glioblastoma/ (accessed on 12 February 2023).
- Zhang, S.; Lou, J.; Li, Y.; Zhou, F.; Yan, Z.; Lyu, X.; Zhao, Y. Recent Progress and Clinical Development of Inhibitors that Block MDM4/p53 Protein–Protein Interactions. J. Med. Chem. 2021, 64, 10621–10640.
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53. Clin. Cancer Res. 2021, 27, 5236–5247.
- Sallman, D.A.; Borate, U.; Cull, E.H.; Donnellan, W.B.; Komrokji, R.S.; Steidl, U.G.; Corvez, M.M.; Payton, M.; Annis, D.A.; Pinchasik, D.; et al. Phase 1/1b Study of the Stapled Peptide ALRN-6924, a Dual Inhibitor of MDMX and MDM2, As Monotherapy or in Combination with Cytarabine for the Treatment of Relapsed/Refractory AML and Advanced MDS with TP53 Wild-Type. Blood 2018, 132, 4066.
- Yang, J.; Zhu, Q.; Wu, Y.; Qu, X.; Liu, H.; Jiang, B.; Ge, D.; Song, X. Utilization of macrocyclic peptides to target protein-protein interactions in cancer. Front. Oncol. 2022, 12, 992171.
- Chang, Y.S.; Graves, B.; Guerlavais, V.; Tovar, C.; Packman, K.; To, K.H.; Olson, K.A.; Kesavan, K.; Gangurde, P.; Mukherjee, A.; et al. Stapled α-helical peptide drug development: A potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl. Acad. Sci. USA 2013, 110, E3445–E3454.
- Zhou, X.; Singh, M.; Sanz Santos, G.; Guerlavais, V.; Carvajal, L.A.; Aivado, M.; Zhan, Y.; Oliveira, M.M.S.; Westerberg, L.S.; Annis, D.A.; et al. Pharmacologic Activation of p53 Triggers Viral Mimicry Response Thereby Abolishing Tumor Immune Evasion and Promoting Antitumor Immunity. Cancer Discov. 2021, 11, 3090–3105.
- Our Approach to Protect Against Chemotherapy-Induced Side Effects. Available online: https://aileronrx.com/our-science/#our-focus (accessed on 13 February 2023).
- Carvajal, L.A.; Neriah, D.B.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 2018, 10, eaao3003.
- Clinical Development. Available online: https://aileronrx.com/clinical-development/ (accessed on 13 February 2023).
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 16, 510–520.
- Dean, A.; Gill, S.; McGregor, M.; Broadbridge, V.; Jarvelainen, H.A.; Price, T.J. 1528P Phase I trial of the first-in-class agent CEND-1 in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer. Ann. Oncol. 2020, 31, S941.
- Pipeline. Available online: https://cendrx.com/pipeline/ (accessed on 13 February 2023).
- Martin-Loeches, I.; Dale, G.E.; Torres, A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti-Infect. Ther. 2018, 16, 259–268.
- Batur, G.; Ermert, P.; Zimmermann, J.; Obrecht, D. Macrocycle therapeutics to treat life-threatening diseases. Chimia 2021, 75, 508.
- Melchers, M.J.; Teague, J.; Warn, P.; Hansen, J.; Bernardini, F.; Wach, A.; Obrecht, D.; Dale, G.E.; Mouton, J.W. Pharmacokinetics and Pharmacodynamics of Murepavadin in Neutropenic Mouse Models. Antimicrob. Agents Chemother. 2019, 63, 10–1128.
- Steinberg, D.A.; Hurst, M.A.; Fujii, C.A.; Kung, A.H.; Ho, J.F.; Cheng, F.C.; Loury, D.J.; Fiddes, J.C. Protegrin-1: A broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742.
- Robinson, J.A.; Shankaramma, S.C.; Jetter, P.; Kienzl, U.; Schwendener, R.A.; Vrijbloed, J.W.; Obrecht, D. Properties and structure–activity studies of cyclic β-hairpin peptidomimetics based on the cationic antimicrobial peptide protegrin I. Biorg. Med. Chem. 2005, 13, 2055–2064.
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.A.; et al. Peptidomimetic Antibiotics Target Outer-Membrane Biogenesis in Pseudomonas aeruginosa. Science 2010, 327, 1010–1013.
- Shankaramma, S.C.; Athanassiou, Z.; Zerbe, O.; Moehle, K.; Mouton, C.; Bernardini, F.; Vrijbloed, J.W.; Obrecht, D.; Robinson, J.A. Macrocyclic Hairpin Mimetics of the Cationic Antimicrobial Peptide Protegrin I: A New Family of Broad-Spectrum Antibiotics. ChemBioChem 2002, 3, 1126–1133.
- Luther, A.; Moehle, K.; Chevalier, E.; Dale, G.; Obrecht, D. Protein epitope mimetic macrocycles as biopharmaceuticals. Curr. Opin. Chem. Biol. 2017, 38, 45–51.
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522.
- Moura, E.C.C.M.; Baeta, T.; Romanelli, A.; Laguri, C.; Martorana, A.M.; Erba, E.; Simorre, J.-P.; Sperandeo, P.; Polissi, A. Thanatin Impairs Lipopolysaccharide Transport Complex Assembly by Targeting LptC–LptA Interaction and Decreasing LptA Stability. Front. Microbiol. 2020, 11, 909.
- Ong, V.; James, K.D.; Smith, S.; Krishnan, B.R. Pharmacokinetics of the Novel Echinocandin CD101 in Multiple Animal Species. Antimicrob. Agents Chemother. 2017, 61, 10–1128.
- Garcia-Effron, G. Rezafungin—Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. J. Fungi 2020, 6, 262.
- James, K.D.; Laudeman, C.P.; Malkar, N.B.; Krishnan, R.; Polowy, K. Structure-Activity Relationships of a Series of Echinocandins and the Discovery of CD101, a Highly Stable and Soluble Echinocandin with Distinctive Pharmacokinetic Properties. Antimicrob. Agents Chemother. 2017, 61, 10–1128.
- Howard, J.F.; Vissing, J.; Gilhus, N.E.; Leite, M.I.; Utsugisawa, K.; Duda, P.W.; Farzaneh-Far, R.; Murai, H.; Wiendl, H. Zilucoplan: An Investigational Complement C5 Inhibitor for the Treatment of Acetylcholine Receptor Autoantibody–Positive Generalized Myasthenia Gravis. Expert Opin. Investig. Drugs 2021, 30, 483–493.
- Duda, P.; Farzaneh-Far, R.; Ma, Z.; Zhu, N.; Thackaberry, E.; Ricardo, A. Neurological Disease Treatment with Zilucoplan. U.S. Patent 17/287,581, 5 May 2022.
- Zilucoplan (RA101495). Available online: https://www.ucb.com/clinical-studies/Clinical-studies-index/Zilucoplan-RA101495 (accessed on 13 February 2023).
- Howard, J.F., Jr.; Nowak, R.J.; Wolfe, G.I.; Freimer, M.L.; Vu, T.H.; Hinton, J.L.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Farzaneh-Far, R.; et al. Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients with Moderate to Severe Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. JAMA Neurol. 2020, 77, 582–592.
- Howard, J.F., Jr.; Bresch, S.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; Maniaol, A.; Mantegazza, R.; et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023, 22, 395–406.
- UCB Announces Positive Data in Myasthenia Gravis with Zilucoplan Phase 3 Study Results. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-positive-data-in-myasthenia-gravis-with-zilucoplan-phase-3-study-results (accessed on 13 February 2023).
- Schmid, B.; Kredel, M.; Ullrich, R.; Krenn, K.; Lucas, R.; Markstaller, K.; Fischer, B.; Kranke, P.; Meybohm, P.; Zwißler, B.; et al. Safety and preliminary efficacy of sequential multiple ascending doses of solnatide to treat pulmonary permeability edema in patients with moderate-to-severe ARDS—A randomized, placebo-controlled, double-blind trial. Trials 2021, 22, 643.
- Schmid, B.; Kranke, P.; Lucas, R.; Meybohm, P.; Zwissler, B.; Frank, S. Safety and preliminary efficacy of sequential multiple ascending doses of solnatide to treat pulmonary permeability edema in patients with moderate to severe ARDS in a randomized, placebo-controlled, double-blind trial: Preliminary evaluation of safety and feasibility in light of the COVID-19 pandemic. Trials 2022, 23, 252.
- Shabbir, W.; Scherbaum-Hazemi, P.; Tzotzos, S.; Fischer, B.; Fischer, H.; Pietschmann, H.; Lucas, R.; Lemmens-Gruber, R. Mechanism of action of novel lung edema therapeutic AP301 by activation of the epithelial sodium channel. Mol. Pharmacol. 2013, 84, 899–910.
- Tzotzos, S.; Fischer, B.; Fischer, H.; Pietschmann, H.; Lucas, R.; Dupré, G.; Lemmens-Gruber, R.; Hazemi, P.; Prymaka, V.; Shabbir, W. AP301, a synthetic peptide mimicking the lectin-like domain of TNF, enhances amiloride-sensitive Na+ current in primary dog, pig and rat alveolar type II cells. Pulm. Pharmacol. Ther. 2013, 26, 356–363.
- Schwameis, R.; Eder, S.; Pietschmann, H.; Fischer, B.; Mascher, H.; Tzotzos, S.; Fischer, H.; Lucas, R.; Zeitlinger, M.; Hermann, R. A FIM study to assess safety and exposure of inhaled single doses of AP301-A specific ENaC channel activator for the treatment of acute lung injury. J. Clin. Pharmacol. 2014, 54, 341–350.
- Krenn, K.; Lucas, R.; Croizé, A.; Boehme, S.; Klein, K.U.; Hermann, R.; Markstaller, K.; Ullrich, R. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: A phase IIa randomized placebo-controlled trial. Crit. Care 2017, 21, 194.
- Hooftman, L.; Chevalier, E.; Wach, A.; Zimmermann, J.; Bruijnzeel, P.; Naue, N.; Heimbeck, I.; Kappeler, D.; Barth, P. WS01.4 A randomised, double-blind, placebo-controlled, parallel-group, dose-escalation study of inhaled single doses of POL6014, a potent and selective reversible inhibitor of human neutrophil elastase (NE), in cystic fibrosis (CF) patients. J. Cyst. Fibros. 2017, 16, S2.
- Taranath, R.; Bourne, G.; Zhao, L.; Frederick, B.; King, C.; Liu, D. Regulation of Iron Homeostasis By PTG-300 Improves Disease Parameters in Mouse Models for Beta-Thalassemia and Hereditary Hemochromatosis. Blood 2019, 134, 3540.
- Taranath, R.; Zhao, L.; Vengalam, J.; Lee, L.; Tang, T.; Dion, C.; Su, A.; Tovera, J.; Bhandari, A.; Cheng, X.; et al. Regulation of Iron Homeostasis and Efficacy of Rusfertide Analog Peptide in a Mouse Model for Polycythemia Vera. Blood 2021, 138, 2006.
- Verstovsek, S.; Kuykendall, A.T.; Hoffman, R.; Ginzburg, Y.; Pemmaraju, N.; Valone, F.; Modi, N.B.; Khanna, S.; O’Connor, P.G.; Gupta, S.K.; et al. A Phase 3 Study of the Hepcidin Mimetic Rusfertide (PTG-300) in Patients with Polycythemia Vera. Blood 2021, 138, 1504.
- Dodd, J.; Yang, W.H.; Makhlina, M. Fr486 ORAL ADMINISTRATION OF THE MELANOCORTIN-1 RECEPTOR AGONIST PL8177 IN A RAT MODEL OF COLITIS. Gastroenterology 2021, 160, S-327.
- Available online: https://docs.publicnow.com/viewDoc?hash_primary=8E226B09BA24D896775EFE0AD9A91AB41D99129D (accessed on 5 April 2023).
- Cary, D.R.; Ohuchi, M.; Reid, P.C.; Masuya, K. Constrained Peptides in Drug Discovery and Development. J. Synth. Org. Chem Jpn. 2017, 75, 1171–1178.
- Culler, M.; Milano, S.; Kurasaki, H.; Tomiyama, T.; Reid, P.; van der Lely, A.J.; Culler, M. ODP353 Sustained Suppression of IGF1 with AZP-3813, a Bicyclic 16-Amino Acid Peptide Antagonist of the Human Growth Hormone Receptor and a Potential New Treatment for Acromegaly. J. Endocr. Soc. 2022, 6, A511.
- Losada, A.; Muñoz-Alonso, M.J.; García, C.; Sánchez-Murcia, P.A.; Martínez-Leal, J.F.; Domínguez, J.M.; Lillo, M.P.; Gago, F.; Galmarini, C.M. Translation Elongation Factor eEF1A2 is a Novel Anticancer Target for the Marine Natural Product Plitidepsin. Sci. Rep. 2016, 6, 35100.
- Wong, R.S.M. Safety and efficacy of pegcetacoplan in paroxysmal nocturnal hemoglobinuria. Ther. Adv. Hematol. 2022, 13, 20406207221114673.
- Our Pipeline. Available online: https://apellis.com/our-science/our-pipeline/ (accessed on 14 February 2023).
- Dijksteel, G.; Ulrich, M.; Middelkoop, E.; Boekema, B. Review: Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 2021, 12, 616979.
- Castañeda, O.; Sotolongo, V.; Amor, A.M.; Stöcklin, R.; Anderson, A.J.; Harvey, A.L.; Engström, Å.; Wernstedt, C.; Karlsson, E. Characterization of a potassium channel toxin from the Caribbean sea anemone Stichodactyla helianthus. Toxicon 1995, 33, 603–613.
- Pennington, M.W.; Chang, S.C.; Chauhan, S.; Huq, R.; Tajhya, R.B.; Chhabra, S.; Norton, R.S.; Beeton, C. Development of Highly Selective Kv1.3-Blocking Peptides Based on the Sea Anemone Peptide ShK. Mar. Drugs 2015, 13, 529–542.
- Tarcha, E.J.; Chi, V.; Muñoz-Elías, E.J.; Bailey, D.; Londono, L.M.; Upadhyay, S.K.; Norton, K.; Banks, A.; Tjong, I.; Nguyen, H.; et al. Durable Pharmacological Responses from the Peptide ShK-186, a Specific Kv1.3 Channel Inhibitor That Suppresses T Cell Mediators of Autoimmune Disease. J. Pharmacol. Exp. Ther. 2012, 342, 642–653.
- Dalazatide. Available online: https://www.kv13therapeutics.com/pipeline/dalazatide/ (accessed on 14 February 2023).
- Schulz-Knappe, P.; Forssmann, K.; Herbst, F.; Hock, D.; Pipkorn, R.; Forssmann, W.G. Isolation and structural analysis of “Urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin. Wochenschr. 1988, 66, 752–759.
- Joseph, S.M.; Cedars, A.M.; Ewald, G.A.; Geltman, E.M.; Mann, D.L. Acute decompensated heart failure: Contemporary medical management. Tex. Heart Inst. J. 2009, 36, 510–520.
- KENTSCH, M.; LUDWIG, D.; DRUMMER, C.; GERZER, R.; MÜLLER-ESCH, G. Haemodynamic and renal effects of urodilatin bolus injections in patients with congestive heart failure. Eur. J. Clin. Investig. 1992, 22, 662–669.
- Mitrovic, V.; Seferovic, P.M.; Simeunovic, D.; Ristic, A.D.; Miric, M.; Moiseyev, V.S.; Kobalava, Z.; Nitsche, K.; Forssmann, W.-G.; Lüss, H.; et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur. Heart J. 2006, 27, 2823.
- Packer, M.; O’Connor, C.; McMurray, J.J.V.; Wittes, J.; Abraham, W.T.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Holcomb, R.; Krum, H.; et al. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N. Engl. J. Med. 2017, 376, 1956–1964.
- Mascio, C.T.; Mortin, L.I.; Howland, K.T.; Van Praagh, A.D.; Zhang, S.; Arya, A.; Chuong, C.L.; Kang, C.; Li, T.; Silverman, J.A. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob. Agents Chemother. 2012, 56, 5023–5030.
This entry is offline, you can click here to edit this entry!
