Multiple sclerosis (MS), neuromyelitis optica (NMO) and myelin oligodendrocyte glycoprotein antibody disease (MOGAD) are inflammatory diseases of the central nervous system (CNS) with a multifactorial aetiology. Environmental factors are important for their development and microorganisms could play a determining role. They can directly damage the CNS, but their interaction with the immune system is even more important. The possible mechanisms involved include molecular mimicry, epitope spreading, bystander activation and the dual cell receptor theory. The role of Epstein–Barr virus (EBV) in MS has been definitely established, since being seropositive is a necessary condition for the onset of MS. EBV interacts with genetic and environmental factors, such as low levels of vitamin D and human endogenous retrovirus (HERV), another microorganism implicated in the disease. Many cases of onset or exacerbation of neuromyelitis optica spectrum disorder (NMOSD) have been described after infection with Mycobacterium tuberculosis, EBV and human immunodeficiency virus; however, no definite association with a virus has been found. A possible role has been suggested for Helicobacter pylori, in particular in individuals with aquaporin 4 antibodies. The onset of MOGAD could occur after an infection, mainly in the monophasic course of the disease.
1. Introduction
Demyelinating diseases of the central nervous system (CNS) are a group of overlapping syndromes characterized by immune-mediated inflammation of the brain and spinal cord. They mainly affect young adult people and their frequency varies worldwide, with multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD) and myelin oligodendrocyte glycoprotein antibody disease (MOGAD) being the most important [
1].
The cause of demyelinating diseases is unknown, but an interaction between genetics and the environment has been established, especially for MS. Among the environmental factors, the main risk factors for MS are vitamin D deficiency, obesity, smoking and infections [
2].
Knowledge of the importance of infectious agents in the triggering of autoimmunity is very old, with MS having been postulated to be triggered by a microorganism as early as 1900 [
3].
The role of persistent slow infections in MS has been suspected since 1975 when elevated titers of antibodies against rubeola, vaccinia and measles viruses were found in the blood and cerebrospinal fluid (CSF) of patients with MS, subacute sclerosing panencephalitis and NMOSD [
4].
In some autoimmune diseases such as MS and type 1 diabetes, infections during pregnancy have also been observed to play a role. In fact, patients with MS exhibit a month of birth seasonality that is different from that of healthy controls, suggesting that perinatal infections could trigger autoimmunity [
5,
6]. Two mechanisms have been postulated to explain the involvement of the fetus during the viral infection affecting the mother. In the first one, the inflammatory immune response caused by the viral infection in the mother is followed by a transmission of cytokines to the fetus. Alternatively, the viruses could be transmitted directly from the mother to the fetus [
7]. On the contrary, there is a possibility of other environmental factors playing a role in the observed impact of the birth season. Specifically, studies have revealed that in the northern hemisphere, this impact correlates with latitude- and climate-related variables such as sunshine duration. Additionally, inadequate levels of vitamin D in the mother may increase the risk of MS in the infant and these levels are closely linked to sun exposure, which varies seasonally [
8].
Viruses can damage the infected organism and trigger autoimmunity in different pathways [
9].
The most plausible mechanism that could explain the role of infections in the triggering of autoimmunity is molecular mimicry, which consists of a cross-reaction between self and nonself epitopes, leading to their presentation by antigen-presenting cells to autoreactive CD4+ T cells [
10,
11].
Other pathways include direct neural toxicity, which is not mediated by inflammation [
9]; bystander activation, which is an abnormal immune activation after tissue damage involving the exposure of normally hidden autoantigens and the production of other autoreactive T cells [
10]; and epitope spreading, which consists of the release of myelin fragments after their disruption in the inflammatory environment, resulting in the exposure of additional epitopes [
12,
13]. Another mechanism is known as “dual T-cell receptors” and involves T cells that carry two different receptors with specificity for myelin and for microorganism epitopes, respectively [
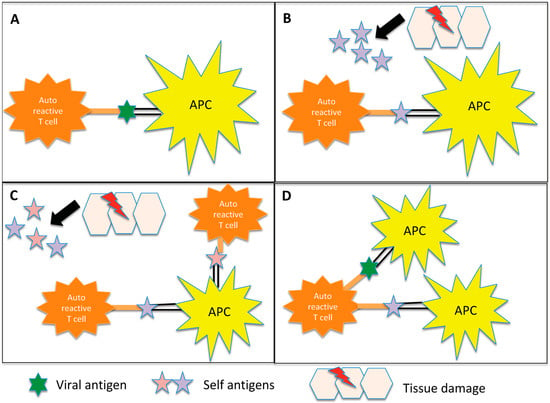
13] (
Figure 1).
Figure 1. Representation of the potential inflammation-mediated mechanisms underlying autoimmunity triggered by infections. (
A) Molecular mimicry: In certain instances, the antigens found on pathogens may bear a resemblance to self-antigens present in the body. This similarity can result in the activation of autoreactive T cells, which erroneously identify self-antigens as foreign. Notably, in demyelinating diseases, antigen-presenting cells (APCs) present myelin components to autoreactive CD4+ T cells, initiating the cascade of autoimmune responses. (
B) Bystander activation: The virus induces a profound inflammatory response, resulting in significant damage to the surrounding tissue. Consequently, additional autoantigens become exposed and antigen-presenting cells (APCs) present these autoantigens to autoreactive CD4+ T cells. (
C) Epitope spreading: Initially, the immune response may target a specific antigen derived from the infecting pathogen. However, as time progresses, the immune response can expand to encompass other self-antigens that share structural similarities or associations with the initial target antigen. In demyelinating diseases, for instance, viral infections can lead to the destruction of oligodendrocytes. Subsequent fragmentation of myelin in the inflammatory milieu exposes additional antigens, contributing to a self-perpetuating cycle of myelin destruction. (
D) Dual T cell receptor: Certain T lymphocytes possess the capability to express multiple T cell receptors, allowing them to recognize both viral and myelin antigens. Consequently, these dual-specificity T cells can activate responses against both types of antigens simultaneously.
2. MS
MS is the most common demyelinating disease of the CNS and its pathogenesis is multifactorial. Indeed, although a genetic predisposition is required, this in itself is not a sufficient condition for its occurrence. Many environmental factors, particularly acting during the first decades of life, are determinants of disease development, among which microorganisms play an important role [
18,
19]. MS is therefore a demyelinating disease for which strong evidence of the role of infections in its aetiopathogenesis is available. Several viruses, including rabies, coronavirus, measles, torque teno virus, herpes viruses and parainfluenza have been hypothesized to be important in MS [
20].
The microorganism with the strongest association with MS is the Epstein–Barr virus (EBV) [
21,
22], but it is possible that more than one agent may be involved [
23]. Moreover, viruses can act as triggers or cofactors and strict interactions have been described among several microorganisms and between them and other environmental and genetic factors, such as among EBV, vitamin D receptor and HERV expression [
9,
24] (
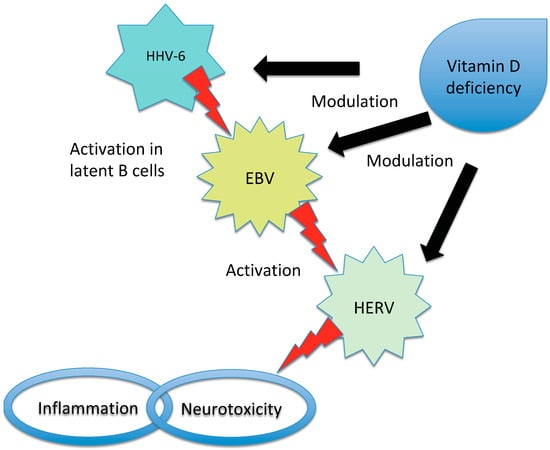
Figure 2).
Figure 2. Representation of the interaction between EBV, HHV-6, HERV and vitamin D in triggering MS. Vitamin D deficiency has been proposed to influence the risk of MS in individuals infected with EBV due to the overlap between the receptor-binding sites of EBNA-2 (a protein produced by EBV) and vitamin D. Additionally, high levels of vitamin D may inhibit EBV infection through apoptosis, while HHV-6 can activate latent EBV in B cells. In patients with MS, an interaction between vitamin D and HERV has also been observed. There is an inverse correlation between HERV-W DNA levels and vitamin D levels and high vitamin D levels may inhibit HERV transactivation. Furthermore, low vitamin D levels increase the risk of MS in individuals with high titers of anti-HHV-6A antibodies. EBV has the ability to induce the expression of certain HERV genes and the activation of HERV-W during infectious mononucleosis has been proposed as a potential effector in the pathogenesis of MS. It is important to note that while these relationships have been identified, the precise mechanisms and their significance in MS development and progression require further investigation. The interplay between EBV, HHV, HERV and vitamin D in the context of MS is complex and multifaceted.
2.1. EBV
The presence of higher titers of antibodies against EBV in patients with MS compared to healthy controls has been understood since the eighties [
25,
26,
27].
This virus is present in almost all patients with MS [
28] and the risk of developing the disease is significantly higher if the patient has experienced a previous mononucleosis infection [
91].
Recently, the role of EBV in the disease has been definitely established by a large cohort study involving more than 10 million military personnel who have been monitored for years [
21]. The authors found EBV seropositivity in all but one of the 801 cases of MS diagnosed during follow-up and the risk of developing the disease for the infected persons was 32-fold higher compared to that for seronegative cases.
Cross-reactivity with myelin basic protein has been detected in the serum of patients with antibodies against epitopes from EBV proteins such as Epstein–Barr nuclear antigen (EBNA)-1 and DNA polymerase [
30,
31].
Despite the detection of EBV-specific oligoclonal bands (OCBs) in the CSF and the finding that OCBs from patients with MS bind to specific EBV proteins [
32,
33,
34], the actual presence of the virus in the CNS of these patients has not been well established and the studies have yielded contradicting results [
92].
The onset of MS may occur decades after the primary EBV infection [
35,
36]. This timeframe reflects the prodromal phase of MS [
93] and it is possible that, during this period, EBV may interact with the immune system of the host through affinity maturation and clonal expansion of B cells, expansion of EBV-infected B cell reservoirs and epitope spreading, thus contributing to MS development [
21].
It has been hypothesized that EBV could act through molecular mimicry, with B cells losing the episomic EBV DNA after replication but retaining the “forbidden” epitope recognition [
94]. According to another theory, EBV could disrupt the blood–brain barrier during primary infection, allowing autoimmune cells to enter the CNS and leading to local inflammation [
95]. The possibility of a persistent EBV infection in the CNS with activation of the immune response and subsequent CNS tissue damage is less probable [
37,
96].
The genetic factors involved in EBV control are becoming increasingly important. The main hypothesis regarding the role of EBV as a “key pathogenic event” in MS involves an altered balance between the virus and the immune system of the host in genetically predisposed individuals [
100]. Indeed, the control of EBV within the human body is influenced by a combination of genetic and environmental factors. These factors, to some extent, are associated with age and sex and can influence the immune response against EBV [
100].
It has been suggested that mutations in EBNA-2 could influence the host response and the risk of developing MS [
38]. Some major histocompatibility complex (MHC) class II alleles predisposing to MS function as coreceptors for EBV entry into B cells [
39], but other MS loci are also involved in the response to EBNA-2 [
100] and there is an interaction between EBNA-1 antibody titers and HLA-DRB1*1501 [
101]. It has been calculated that the risk of MS is more than 20-fold higher if HLA-DRB1*1501 is present in combination with high titers of EBNA-1 antibodies [
40].
Nevertheless, there is evidence of DMTs, such as interferon beta, teriflunomide and ocrelizumab, playing a role in decreasing the immune response to EBV or inhibiting its replication in “in vitro” studies [
44,
45]. Therefore, the effectiveness of DMTs could be partly attributed to their capacity to modify the interaction between EBV and the immune system of the host.
2.2. HERV
HERV are a class of retroviruses incorporated in the human genome millions of years ago and they represent up to 8% of the human genome. HERV are classified into various classes and families, with each family being designated by appending a letter after the acronym “HERV”. The letter corresponds to the transfer RNA specificity of the primer-binding site. The activation of the K, H and W types by a variety of different stimuli (i.e., infection with EBV, human herpes virus-6 and other viruses) could trigger demyelination in MS [
107,
108,
109]. It is estimated that 8% of the entire human genome is constituted by some types of HERV, which normally have a regulatory function for human gene expression [
110].
HERV-W is the type most closely implicated in MS pathogenesis and its envelope protein has been detected in the serum, brain, perivascular infiltrates and macrophages of patients with MS [
46,
47,
48]. Moreover, HERV mRNA has been found in the brain lesions, CSF and blood cells of individuals with MS [
49,
50]. It has been demonstrated that the HERV-W envelope can stimulate microglia to damage myelinated axons, activate monocytes and endothelial cells and drive the production of pro-inflammatory cytokines responsible for demyelination and neurodegeneration [
24,
48].
HERV-W envelope epitopes are expressed on activated B cells and monocytes in individuals with MS, suggesting cross-reactivity through molecular mimicry [
51,
53]. The HERV-W envelope protein can be colocalized with oligodendrocyte progenitors in normal white matter, resulting in disrupted myelin repair, abnormal demyelination and development of MS [
54].
Other HERV types, such as HERV-K and -H, have been reported to be associated with MS. In particular, the levels of some HERV-K gag genes are increased in mononuclear cells and in the brains of individuals with MS [
52,
111]. The HERV-H envelope and gag proteins have been reported to be present in the serum of MS patients [
55] and when patients with active disease were compared to those with inactive MS and with healthy controls, both the HERV-H envelope protein and an HERV-H single nucleotide polymorphisms were found only in immune blood cells derived from the first group [
51,
52].
HERV-W appears to be implicated not only in the pathogenesis of MS, but also in its clinical course. Indeed, patients with a high level of disability or those in phases of high disease activity have more active loci for the HERV-W envelope compared to other patients with MS and to healthy controls [
20].
2.3. Human Herpes Virus (HHV)
Higher titers of antibodies against herpes simplex virus-2 have been reported in patients with MS, whereas herpes simplex virus-1 has been reported in pediatric cases and in CIS, but not in adult MS [
115].
The HHV subtype with stronger evidence of implication in MS is HHV-6. HHV-6 infects the majority of the population before one year of age and comprises two species: HHV-6A and HHV-6B [
116]. The detection of HHV-6 DNA in MS lesions, CSF and blood cells, the observation of high antibody titers in patients with MS and the identification of OCB specificity against HHV-6 have suggested an association between infection with this microorganism and the disease [
63,
64].
Although other studies have reported no association between HHV-6 and MS risk [
117], a clear difference between patients with MS and healthy controls has been established after a thorough metanalysis in terms of anti-HHV-6 antibody titers and HHV-6 mRNA and DNA [
118].
2.4. Gut Microbiota
There is clear evidence of a link between the gut microbiota and MS, with both bacteria and fungi playing an important role [
122,
123]. Different microorganisms comprise the gut microbiota, with
Firmicutes, Bacteroidetes, Proteobacteria and
Actinobacteria representing more than 97% of it [
124]. Its composition is influenced by several internal and external factors, including diet, lifestyle and physical activity [
125]. Disruption in the normal composition of gut microbiota, known as “dysbiosis”, has been related to many diseases [
126]. The complex interplay between the CNS and the gut has led to the concept of the “gut-brain axis” and it has been noted that the development of the immune system from birth depends on the composition of the gut microbiota [
127]. The gut microbiota of individuals with MS has some peculiarities compared to that of healthy individuals, such as an increased abundance of
Akkermansia, Ruminococcus, Blautia and
Bifidobacterium, while other microorganisms such as
Bacteroides, Parabacteroides, Prevotella and
Lactobacillus are less represented [
128].
There is evidence of the amelioration of MS symptoms through the manipulation of the gut microbiota composition with probiotic supplementation, the use of antibiotics or changes in dietary habits. Moreover, some pilot studies have confirmed the promising role of microbiota transplantation [
129].
It has recently been suggested that both DMTs and vitamin D may have an impact on the gut microbiota [
130]. Indeed, dimethylfumarate can promote an increase in
Bacteroides and a decrease in two clostridial families, whereas interferon beta can cause an increase in
Prevotella [
73,
74].
2.5. Fungi
Recent studies have shown that fungi may play an important role in the pathogenesis of MS [
14] and the finding that genes involved in innate immunity are associated with the disease suggests a link between MS and dysregulation of the innate immune system [
18].
Candida albicans is the fungus most closely associated with MS according to the available evidence.
Candida infection is more frequent in MS patients compared to healthy individuals and correlates with disease severity. Antibodies against
Candida have also been found in the CSF of individuals with MS [
77,
78,
79]. This association may be the result of molecular mimicry, with memory B cells recognizing both
Candida and CNS epitopes [
17]. It has been hypothesized that the higher prevalence of MS among women could be partially explained by the higher titers of antibodies against
Candida present in healthy women compared to men [
132].
Notably, T helper 17 cells play a crucial role in MS pathogenesis and they are also important for antifungal immunity [
134,
135].
Candida albicans mannoproteins promote the production of interleukin 17 (IL-17) and even a low fungal load inside the CNS can trigger high levels of a T helper-17-mediated immune response, thus provoking an important increase in IL-17 [
136,
137].
2.6. Mycobacterium avium Subspecies Paratuberculosis (MAP)
To date, this microorganism has been associated with MS only in Sardinia and Japan [
82,
84]. In a large cohort of Sardinian MS patients, a significantly higher frequency of anti-MAP antibodies and MAP DNA was observed compared to healthy controls [
82,
83]. Moreover, specific MAP antigens that are homologous with MS-related proteins have been described, suggesting that molecular mimicry and the ability to enhance the immune response are the main pathogenic mechanisms linking MAP to MS development [
31,
85,
86,
138].
After the study on the Sardinian cohort, a higher response against MAP was observed in a cohort of Japanese MS patients compared to healthy controls [
84]. The prevalence of anti-MAP antibodies in the Sardinian and Japanese cohorts was quite similar [
16].
MAP infection can enhance the risk of MS if other factors, such as genetic predisposition, are present [
15]. Nevertheless, no specific association between the predisposing haplotypes and MAP positivity was found in the Sardinian population. On the other hand, lower titers of anti-MAP antibodies have been detected in patients carrying at least one protective haplotype [
87].
It has been suggested that the beneficial effects of the Bacillus Calmette–Guerin (BCG) vaccine in MS patients are derived from MAP mitigation [
139]. On the other hand, the vaccine should prevent neuroinflammation, contrasting the low exposure to microbes and other pro-inflammatory changes in lifestyle that have occurred in recent decades [
140].
2.7. CMV
The active role of CMV in the pathogenesis of MS has been hypothesized [
88] and it has been clearly established that it has a protective effect against the disease [
22,
26,
65,
90]. This may be related to the capacity of CMV to enhance the immune control of EBV infection, thus reducing the risk of MS development [
144].
3. NMOSD
NMOSD designates a group of demyelinating syndromes characterized by specific clinical and radiological findings in addition to the presence of anti-aquaporin-4 (AQP4) antibodies in 80–90% of the patients [
145]. The prevalence of the disease is increasing, probably because of changes in environmental factors such as the increased use of antibiotics and improvements in hygiene conditions [
146].
NMO pathogenesis involves both genetic and environmental factors; however, clear evidence is lacking at the moment. It is likely that factors in the environment trigger the onset of the disease in genetically predisposed individuals [
147].
The evidence regarding the role of infections in NMO is not as strong as that available for MS due to the dearth of experimental data, which in turn may be the result of the low prevalence of the disease and the small sample size of the studies. Nevertheless, several infectious agents have been described as potential triggers for disease onset or exacerbation, including CMV, VZV, EBV, hepatitis A virus, human immunodeficiency virus (HIV),
Mycobacterium tuberculosis,
Mycoplasma pneumoniae, human T-lymphotropic virus type 1 and dengue virus [
148,
149].
3.1. Tuberculosis (TB)
Many cases of NMO have been reported after or during active TB, in particular with pulmonary involvement [
155,
158,
160]. Notably, Rafai et al. reported on two patients with NMOSD, each presenting distinct clinical observations. In one case, the diagnosis of pulmonary TB closely preceded the onset of NMOSD, whereas in the other patient, neurological symptoms of NMOSD were observed prior to the detection of pulmonary TB positivity in the sputum [
158]. An additional remarkable case involved a patient who consistently experienced NMOSD relapses following episodes of active pulmonary TB [
155].
3.2. Helicobacter pylori (H. pylori)
Contradictory results have been reported in studies regarding the prevalence of this microorganism in patients with NMO.
When AQP4-positive and AQP4-negative NMOSD patients were compared, higher titers of antibodies against
H. pylori were observed only in the former group [
161]. In contrast, another study evaluating patients with NMO found high levels of antibodies against
H. pylori in both AQP4-positive and AQP4-negative patients when compared to patients with MS and to healthy controls, although the stronger association was with the AQP4-positive group [
162]. In another cohort, a higher prevalence of
H. pylori infection was described in AQP4-positive compared to AQP4-negative patients [
163].
3.3. EBV
Data regarding the active role of EBV in NMOSD are not as robust as those in MS. However, the serum and CSF titers of anti-early antigen (EA) IgG antibodies are higher in patients with NMOSD than in those with MS or in healthy controls and they are positively associated with AQP4 levels [
165]. The same study showed that anti-EA IgM levels were higher in NMO and MS patients compared to healthy controls, whereas antiviral capsid antigen and anti-EBNA IgG levels were higher in MS patients than in NMO patients or healthy individuals. It has been suggested that pro-inflammatory molecules produced early during EBV infection may exacerbate NMO. The high levels of anti-EA antibodies could indicate active viral replication in patients with NMO and the presence of these antibodies in the CSF is indicative of their local production [
163].
3.4. HERV
Despite the limited number of studies, there is a general agreement regarding the negative association between antibodies against the HERV-W envelope and NMOSD, particularly in patients that are seropositive for AQP4 IgG. In fact, patients with NMOSD have lower levels of antibodies compared to patients with MS and to healthy individuals [
167,
168]. The different trends in the levels of these antibodies among patients with MS and NMOSD suggest that this could be used as a potential biomarker to improve discrimination between these two diseases [
167].
3.5. Gut Microbiota
The roles of
Clostridium perfringens and
Escherichia coli in NMOSD have been suggested by their effect in T helper 17 and T regulatory cell balance [
147]. Moreover, the presence of
Clostridium perfringens has been found to be enhanced in individuals with NMO compared to that in patients with MS and healthy controls [
169] and potential molecular mimicry between this microorganism and AQP4 has been suggested [
170].
3.6. Fungi
There is no clear evidence supporting the role of fungi in NMO; however,
Candida albicans has been reported to promote lymphocyte proliferation in these patients, suggesting a possible exacerbation of NMO after fungal infection [
148].
3.7. Human Immunodeficiency Virus (HIV)
A separate discussion needs to be conducted regarding HIV infection associated with NMOSD, an entity that has been recognized only relatively recently. To date, seven cases of HIV-NMOSD have been described worldwide, including both AQP4-positive and AQP4-negative patients and the peculiarity of this disease is the concomitant presence in the same patients of hypo- and hyperactivation of the immune system [
171]. The mechanism of autoimmunity during HIV infection is unclear; however, autoimmune disease may occur when immune competence is still present around the time of seroconversion or during retroviral therapy.
4. MOGAD
The term “MOGAD” encompasses a series of demyelinating diseases of the CNS characterized by optic neuritis, optic neuromyelitis, myelitis, brain/brainstem syndrome or acute disseminating encephalomyelitis (ADEM) together with the presence of anti-MOG IgG antibodies in serum [
177,
178].
It should be noted that the presence of anti-MOG antibodies can be transient and the disease is sometimes monophasic [
179]. Moreover, low antibody titers have a lower predictive value and may indicate a false positive [
180].
ADEM is the most common manifestation in the pediatric population and often occurs after infection with EBV, measles, influenza, enterovirus, coronavirus, herpes simplex and, rarely,
Mycoplasma pneumoniae [
181,
182,
183,
184,
185,
186,
187,
188,
189,
190]. Recently, it has been observed that previous infection is more common during the monophasic course of the disease [
191].
How microorganisms damage the CNS is still not clear. Both direct and immune-mediated mechanisms have been proposed [
190].
5. What about SARS-CoV-2?
In recent years, after the beginning of the COVID-19 pandemic, several cases of post-infectious demyelinating diseases have been reported worldwide.
SARS-CoV-2 has direct neurotropism, but its DNA has never been found in the CSF of reported cases; thus, direct damage is not the probable mechanism [
196]. On the other hand, the virus can cause an aberrant immune response, increasing the levels of cytokines and chemokines and inhibiting regulatory T cells [
197,
198]. This mechanism may be related to both cases of relapses and of new-onset demyelinating diseases.
6. Conclusions
Infectious factors, including viruses, fungi and bacteria, have the potential to act as potent triggers of autoimmunity, playing a significant role in the initiation of demyelinating diseases and their subsequent clinical manifestations.
Currently, it is clear that MS occurs in genetically predisposed individuals when particular environmental factors are present and that previous EBV infection has a determinant role in the pathogenesis of the disease. Indeed, the risk of MS is very low in patients that are EBV seronegative and increases after EBV infection, especially in genetically predisposed individuals. Among the remaining microorganisms that have been associated with the disease, HERV-W has the more important role and it has been considered the missing link between EBV and other environmental factors and the development of MS.
The evidence for the role of microorganisms in the pathogenesis of NMOSD is still not as clear and solid as that available for MS. However, several viruses and bacteria have been associated with the onset or exacerbation of the disease. It is likely that no specific infectious agents are directly responsible for the triggering of NMOSD symptoms. A more probable hypothesis is that any infection that derives in immune system imbalance, causing over-reactivity, production of self-reactive lymphocytes and AQP4, enhanced levels of cytokines and damage to the blood–brain barrier may be involved in the onset or exacerbation of NMOSD.
This entry is adapted from the peer-reviewed paper 10.3390/life13061309