Lignocellulosic biomass is an attractive natural resource because of its abundance, renewability, recyclability, and low cost. The ever-increasing developments in nanotechnology have opened up new vistas in sensor fabrication such as biosensor design for electronics, communication, automobile, optical products, packaging, textile, biomedical, and tissue engineering. Due to their outstanding properties such as biodegradability, biocompatibility, non-toxicity, improved electrical and thermal conductivity, high physical and mechanical properties, high surface area and catalytic activity, lignocellulosic bionanomaterials including nanocellulose and nanolignin emerge as very promising raw materials to be used in the development of high-impact biosensors.
1. Introduction
The aim of generating an eco-friendly and sustainable world has gradually increased the request for renewable bio-based natural sources on a global scale. Non-renewable fossil resources are not sustainable as well as they have negative environmental influences. For this reason, environmentally-friendly alternative resources have long been sought to replace the fossil [
1]. Bio-based raw materials such as forest and agricultural wastes, wood, crops, and food residues have been widely considered as appropriate benign renewable resources that can be utilized to manufacture new generation value-added materials. The plants consist of lignocellulosic structures involving cellulose (40–50%), hemicellulose (20–40%) and lignin (20–30%) are known to be the most common and accessible bio-resources on the planet [
2].
Being the most abundant natural polymer on earth with an annual production of about 10
11–10
12 tons, cellulose has a great value and potential for obtaining eco-friendly bio-based products [
3]. Coniferous and deciduous trees, annual plants such as bamboo, bagasse, raphia palm, etc., and also most agricultural residues and sea creatures known as tunicate as well as some bacteria and fungi can be considered cellulose sources [
4]. In 1838, Payen was the first to identify a major insoluble waste called cellulose. Since its discovery, thousands of scientific papers, patents, and books have been published concerning the importance of this natural polymer [
5]. Cellulose (C
6H
10O
5)
n is a high molecular weight homopolysaccharide constituted of β-1,4-anhydro-d-glucopyranose units (AGUs), and the glucose rings rotated through an angle of 180° about the molecular axis and hydroxyl groups in an equatorial position [
6]. In this way, the two neighboring glucose rings form the auxiliary unit called cellobiose [
7]. It is packed into microfibrils that are kept together by intra- and inter-molecular hydrogen bonds as well as intermolecular van der Waals forces with both non-reducing and reducing ends [
8]. These intra- and inter-chain non-covalent attractions are important for the stability and firm structure of cellulose, the latter of which is basic for plants and some marine creatures [
9]. Furthermore, the two ends of cellulose, each AGU has one primary hydroxyl group on C6 and two secondary hydroxyl groups on C2 and C3. A large number of hydroxyl groups make cellulose molecules easy to compose intermolecular or intramolecular hydrogen bonds. It is noteworthy that the 1HO(2) and 1HO(6) hydroxyl groups of the forming glucose units act as hydrogen-bond donors to water, whereas the 1HO(3) groups behave exclusively as hydrogen-bond acceptors from water and donate hydrogen to their intra-chain neighbors O(5) [
10,
11]. The presence of the strong hydrogen bonding clarifies it with the crystalline nature, rigidity, and un-reactiveness toward water and other chemicals [
12]. On the one hand, the weak hydrogen bonds present in amorphous zones demonstrates hydrophilicity, accessibility, and flexibility. The structure of cellulose establishes the features such as degradability, hydrophilicity, chemical variability, and chirality owing to the presence of OH groups [
13].
The essential drawback of the pristine cellulose is that it is difficult to process owing to the fact that strong intramolecular and intermolecular interactions make it very difficult to dissolve in typical organic solvents. Due to their outstanding dissolving capabilities, cellulose derivatives are potential alternatives to pristine cellulose. Ethanolic or aqueous hydrolysis can also be utilized to transform them to cellulose. Cellulose ethers (methyl cellulose, ethyl cellulose, and carboxymethyl cellulose), cellulose esters (cellulose acetate), cellulose nitrate, and cellulose sulfate are the most well-known cellulose derivatives [
14]. Cellulose acetate is a renewable material that has gradually attracted considerable research interest because of its potential benefits such as being biocompatible, non-toxic, non-corrosive, and biodegradable. It is usually used as a dispersant to equally distribute nanoparticles in a suspension [
15].
Lignin is a three-dimensional and quite complex phenolic chemical compound and, after cellulose, is the most widely available polymer in the earth [
16,
17,
18,
19]. Due to its complex structure, lignin occurs in significant quantities as a by-product of the pulp industry and bioethanol manufacturing processes, but only a few of them are currently used at an industrial level [
16,
20,
21,
22,
23]. The characteristics of lignin show changes based on the type of lignocellulosic raw material obtained, such as softwood, hardwood, or annual plant [
24]. Furthermore, pulping conditions, isolation methods, and other procedures applied to lignocellulosic raw materials will provide lignin with different properties and structures. This diversity and complexity directly affect the usability of lignin in many potential applications. However, regardless of the source and production method, all types of lignin have chemical functional groups that are adaptable to modifications for many different purposes, such as aliphatic and aromatic hydroxyl, carbonyl, methoxyl, as well as phenyl groups [
17,
25].
2. Lignocellulosic Material Based Sensors
2.1. Cellulose Based Sensors
Our world is surrounded by electronic devices which have key roles in our daily lives. Most of these electronic devices such as portable computers, digital cameras, mobile phones, tablets, electronic watches, and Bluetooth speakers are equipped with sensors. Sensors are analytical instruments that respond to different stimulating factors and are situated to recognize external factors for example light, motion, humidity, temperature, sound, or chemicals [
95]. Sensors have been categorized by the International Union of Pure and Applied Chemistry (IUPAC) into three groups: physical sensors, chemical sensors and biosensors [
96]. Sensor technologies have attracted significant notice in the application of different areas such as health, environment, and industry [
97]. Notably, while people are using these technological devices they could choose the user-friendly, economical, sensitive, and excellently error-free detection tools [
98,
99,
100].
“Green electronics” which are fabricated from natural materials, especially cellulose and its derivatives came into prominence in order to obtain the sustainability of electronics. Cellulose represents the total annual biomass production which is about 1.5 trillion tons, which is considered as an almost endless source of raw material for the increasing request for environmentally friendly and biocompatible products [
101]. Nanocellulose-based materials with excellent electrical, optical, and mechanical properties have become an alternative to their commercial counterparts in various sensing applications such as environmental monitoring, food safety, physical sensing, human disease detection, and healthcare.
With the use of nanomaterials with advanced physicochemical properties, breakthrough developments have occurred in sensor technology in the last decade. However, due to their toxic effects on the environment and human health, research efforts have been focused on those types of nanomaterials generated from biomaterials [
102,
103,
104]. Nowadays, the development of sensors based on nanomaterials especially nanocellulose has attracted enormous attention in the biomedical area for monitoring and managing human health [
105,
106,
107]. It should be noted that nanocellulose-based materials generally exhibit excellent mechanical, thermal, chemical, physical, and barrier properties and they are chemically inert [
108]. Concerning the considered applications, different modifications are available for the surface of nanocellulose supported with hydroxyl groups by introducing special functional groups.
2.2. Hemicellulose Based Sensors
Hemicelluloses are biopolymers with multifunctional properties for biosensor utilization [
160]. Hemicellulose has been reported to be used in drug delivery, tissue engineering, electronic skins (e-skins), human-machine interfaces, health monitoring, cancer chemotherapy, biosurfactant chemistry, metal ions films, hydrogels, conductive polymers, artificial intelligence applications, and dye adsorption.
2.3. Lignin Based Sensors
2.3.1. Lignin Based Physical and Chemical Sensors
Recently, there has been an increasing series of studies highlighting the significant potential applications of lignin. Lignin has found applications in various fields such as sunscreen agents, drug distribution, functional fillers, abrasion protection, and energy storage. Its non-toxicity, biodegradability, good mechanical properties, and other qualities make lignin a popular raw material for physical sensors. Ongoing research focuses on the preparation of sensors with high lignin content and multifunctional properties.
Han et al. [
175] fabricated a polyvinyl alcohol (PVA) hydrogel integrated with lignin-silver hybrid nanomaterials, demonstrating remarkable compressibility. The lignin-silver hybrid nanomaterials acted as strong modifiers of the hydrogel, providing powerful hydrogen bonds and facilitating electron shift. By harnessing these exceptional characteristics, the PVA/lignin-silver hybrid nanomaterial hydrogel holds great potential as a pressure-sensitive sensor for monitoring signals. The demethylation process of lignin led to the liberation of phenolic hydroxyl groups, which enhanced the adhesive properties of lignin and improved its reducibility [
176].
Chen et al. [
177] conducted a study where they applied biodegradable and renewable lignin material onto the surface of a metal electrode to create humidity-sensitive sensors. The sensing productivity for humidity detection of lignin-based quartz crystal microbalance (QCM) sensors were investigated using both symmetric and ringed electrode configurations, employing the swing circuit method. Based on the experimental findings, it was observed that the humidity sensitivity of the QCM sensor utilizing a ringed electrode configuration (61 Hz/%RH) was greater than that of the sensor employing a symmetric electrode configuration within the RH range of 11.3% to 97.3%. The underlying mechanism responsible for the improved sensitivity of the QCM humidity sensor, which is based on lignin and features a ringed electrode configuration, was examined and explained through the utilization of equivalent electronic circuit analysis and simulation methods. The findings of this study provide strong evidence supporting the suitability of lignin as a highly effective material for humidity detection. Furthermore, the optimization of the electrode structure configuration utilizing the fringing field effect emerges as a promising strategy for enhancing the humidity sensitivity of QCM sensors.
In this research, Sun et al. [
178] presented a simple procedure for synthesizing lignin-based carbon dots (L-CDs). The raw materials used in the synthesis process included lignin, citric acid, and ethylenediamine. The researchers optimized the synthesis conditions to enhance the fluorescence lifetime of the L-CDs. Furthermore, the structure and pH-responsive characteristics of the lignin-based carbon dots were thoroughly examined in this study. By combining L-CDs, N-isopropylacrylamide (NIPAM), and polyvinyl alcohol (PVA), the researchers successfully synthesized fluorescent hydrogels that exhibited pH/temperature dual response through free radical polymerization. The diameter of the L-CDs ranged from 2 to 5 nm, and they exhibited a crystalline structure resembling graphene. Under the optimized conditions, the L-CDs exhibited a fluorescence lifetime of approximately 12 ns and a quantum yield of 43.9%. Within the pH range of 1 to 10, the fluorescence intensity of the L-CDs exhibited a proportional relationship with the pH value. Furthermore, researchers synthesized a pH/temperature dual-responsive hydrogel by incorporating L-CDs. The hydrogel demonstrated a higher value of elastic modulus (G′) in comparison to the viscous modulus (G″). They were also noted that the temperature sensibility and water retention rate of the hydrogel gradually declined as the PVA content exceeded 10 wt%.
Due to their favorable characteristics, lignin-based materials have garnered significant interest as sensing materials in recent studies. Lignin-based materials exhibit high thermal stability, making them suitable for applications that involve elevated temperatures. They also possess strong UV absorption capabilities, which can be advantageous in sensing applications that require protection from UV radiation. Additionally, lignin-based materials exhibit excellent water stability, maintaining their structural integrity even in humid or aqueous environments. These materials offer similar attractive morphological and mechanical qualities, such as flexibility and mechanical strength, which are desirable for sensor development. Furthermore, lignin-based materials are considered low-cost and sustainable, aligning with the growing demand for environmentally friendly and economically viable sensing solutions. In general, lignin-based materials show great potential for the advancement of innovative detecting materials with exceptional performance attributes.
In a study carried out by Joshi et al. [
179], the synthesis of zinc oxide (ZnO) nanorods with a hierarchical-type structure was achieved using fragmented lignin. The researchers isolated lignin from cossene using a microwave-assisted procedure and fragmented it under alkaline conditions with the addition of hydrogen peroxide. Subsequently, the disjoined lignin was utilized as a pattern for synthesizing the zinc oxide (ZnO) nanorods. Powder X-ray diffraction (XRD) analysis was conducted to analyze the resulting ZnO samples, which revealed a hexagonal structure. In comparison to ZnO instances without disjoined lignin, the presence of disjoined lignin resulted in the formation of a self-assembled hierarchical nanostructure, comprising nanorods with lengths ranging from 200 to 500 nm and a diameter of 30 nm. The inclusion of disjoined lignin had a notable impact on the extent and morphology of the ZnO nanoparticles, ultimately giving rise to the observed hierarchical structure.
2.3.2. Lignin Based Biosensors
Lignins have gained significant attention in several areas such as electrochemistry, pharmacy, sensors, and biomedicine, because of their versatile applications. Although the utilization of lignin as a biosensor for medical or bacterial sensing is still relatively uncommon, there are researchers actively exploring the potential of lignin-based biosensors.
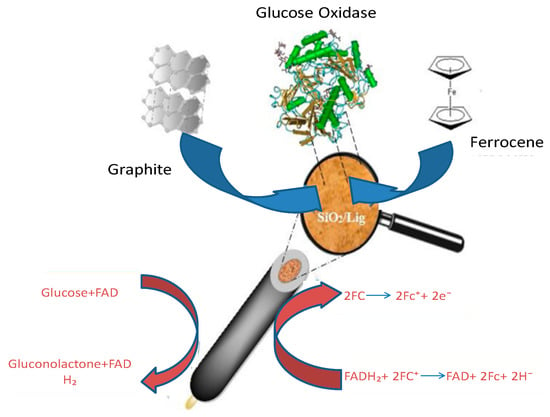
Jędrzak et al. [
180] conducted a study in which they introduced a new method for fabricating an enzyme biosensor utilizing an affordable and functional silica/lignin (SiO₂/Lig) hybrid material. The researchers utilized a functional biohybrid SiO₂/Lig material as a platform for immobilizing glucose oxidase (GOx) through absorption on that surface. Mechanisms involved in the immobilization process are illustrated in
Figure 1.
Figure 1. The acting of GOx-SiO2/Lig into CPE for β-d-glucose (Reproduced from Jędrzak et al. [
180]).
The immobilized quantity of Gox in the SiO₂/Lig composite was 25.28 mg g⁻1, exhibiting twice the amount compared to its presence on non-functionalized SiO₂. The GOx-SiO₂/Lig system was integrated with single-walled carbon nanotubes and platinum nanoparticles as a supportive framework for the development of an AI-generated glucose biosensor. Additionally, the ferrocene redox-mediated GOx-SiO₂/Lig-based carbon paste electrode was assessed as an active ingredient in the second descendants’ glucose biosensor. The findings suggest that GOx-SiO₂/Lig could be the preferred material for developing an efficient and cost-effective biosensor that can be utilized in various electrode configurations.
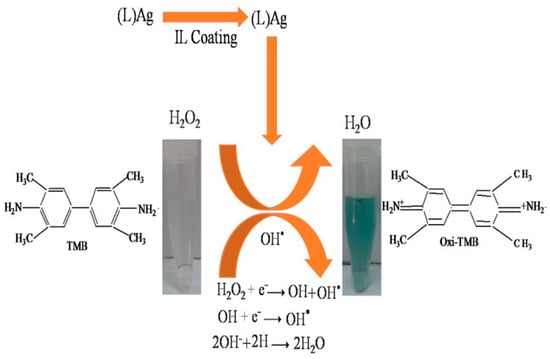
Nishan et al. [
181] conducted a study where they utilized lignin as a stabilizer and reductive agent to prepare silver nanoparticles (AgNPs). The synthesized AgNPs were subjected to a coating process using an ionic liquid (1-H-3-methylimidazolium acetate) to produce ionic liquid-coated lignin stabilized silver nanoparticles (LAgNPs). The application of the coating resulted in improved catalytic activity, immobility, conductibility, and diffusibility of the nanoparticles, thereby enabling their effective utilization as peroxidase mimics for the colorimetric detection of hydrogen peroxide (H₂O₂). The reaction mechanisms involved in this process were depicted in
Figure 2. The developed protocol involved the combination of ionic liquid-coated nanoparticles (IL-NPs) with a solution of 3,3′,5,5′-tetramethyl benzidine (TMB) and a wadding solution to create a sensor that detects hydrogen peroxide (H₂O₂) based on colorimetric principles. In optimized conditions, the sensor demonstrated excellent performance with a wide linear range (1 × 10⁻⁹–3.6 × 10⁻⁷ M), a low detection limit of 1.37 × 10⁻⁸ M, and a quantification limit of 4.59 × 10⁻⁸ M, with an R
2 value of 0.999. The suggested sensing probe presents a straightforward, fast, high degree of sensitivity, selector, and stable biomimetic catalyst approach for colorimetric detection of hydrogen peroxide (H₂O₂), with potential applications in medical diagnostics. The sensor has demonstrated its selectivity in detecting hydrogen peroxide even in the existence of other concurrent substances. Furthermore, it has been effectively employed in the sensing of H₂O₂ in real examples.
Figure 2. Proposed reaction mechanism for the detection of H
2O
2 (Reproduced from Nishan et al. [
181]).
Lastly, tuberculosis is a highly contagious disease caused by Mycobacterium tuberculosis, and its accurate and timely diagnosis remains a challenge. Current diagnostic methods are limited in their sensitivity and time-consuming nature. In a study by Tai et al. [
182], a green graphene nanofiber laser biosensor (LSG-NF) decorated with synthetic silver nanoparticles (AgNPs) obtained from lignin extracted from palm oil was developed. To affirm the sensing capability, a selective DNA sample was captured on AgNPs and surveyed for specific bonding to Mycobacterium tuberculosis target DNA through selective hybridization and mismatch analysis. Successful immobilization and hybridization of DNA were confirmed through the identification of phosphorus and nitrogen signals using X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) analyses were conducted. The analysis demonstrated good replicability and stability. This approximation presents a potential and cost-effective sensing system for the detection of Mycobacterium tuberculosis biomarkers, providing a novel avenue in medical diagnosis. By harnessing lignin as a key component in the synthesis of synthetic silver nanoparticles, this study emphasizes the capacity of lignin as a valuable material in advancing the field of biosensors for disease detection.
This entry is adapted from the peer-reviewed paper 10.3390/mi14071450